Abstract
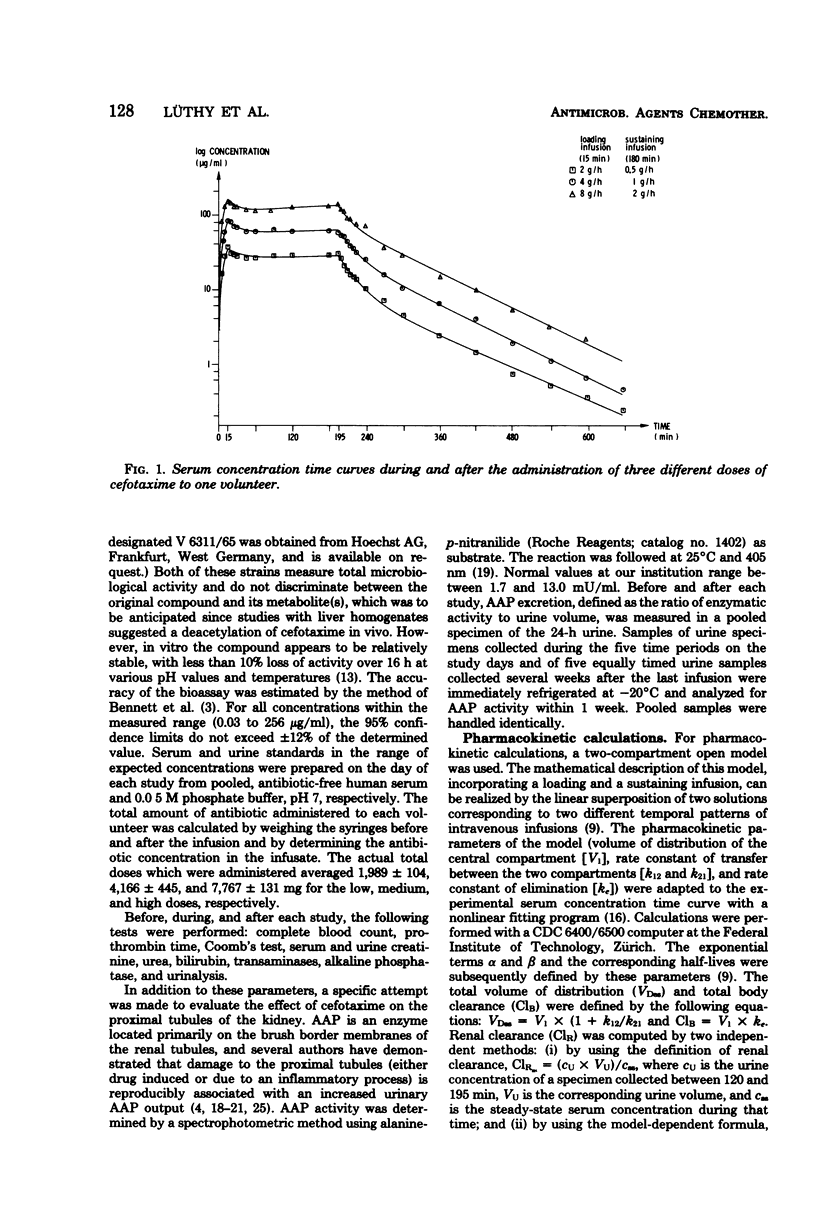
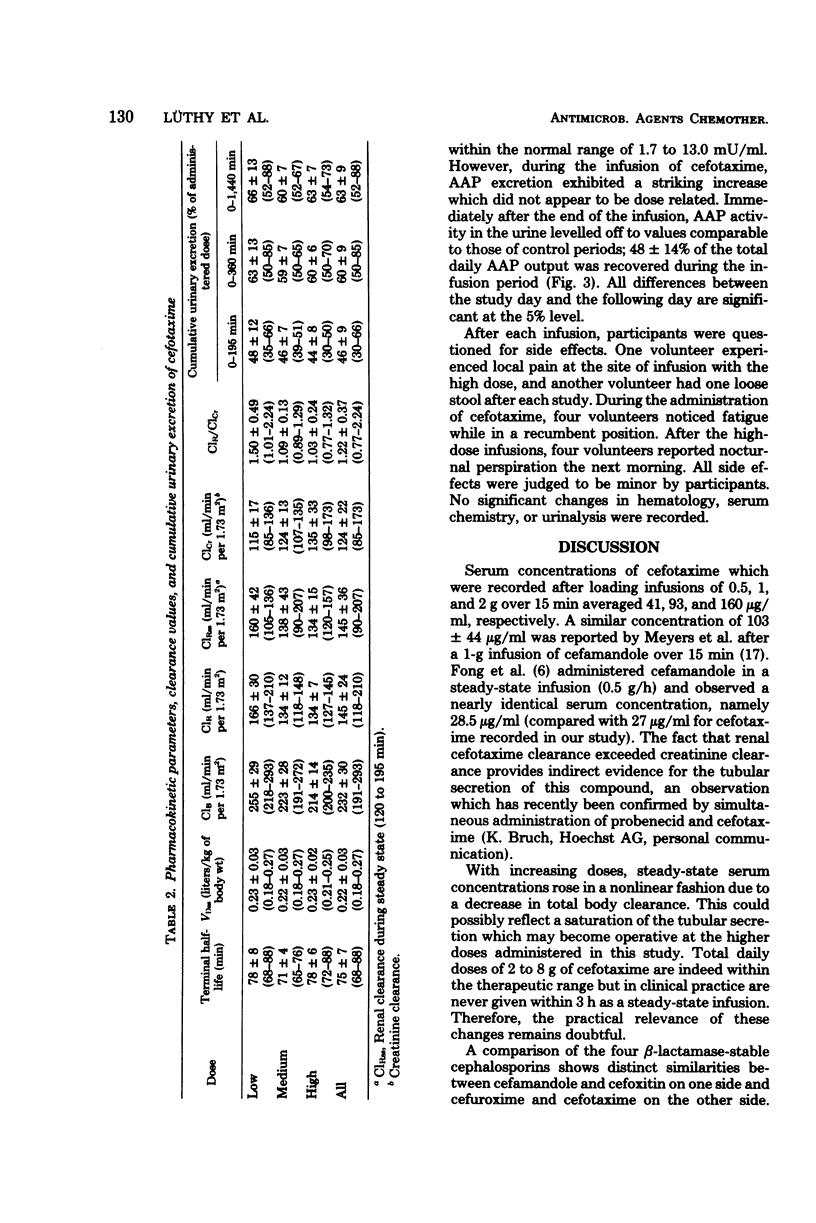
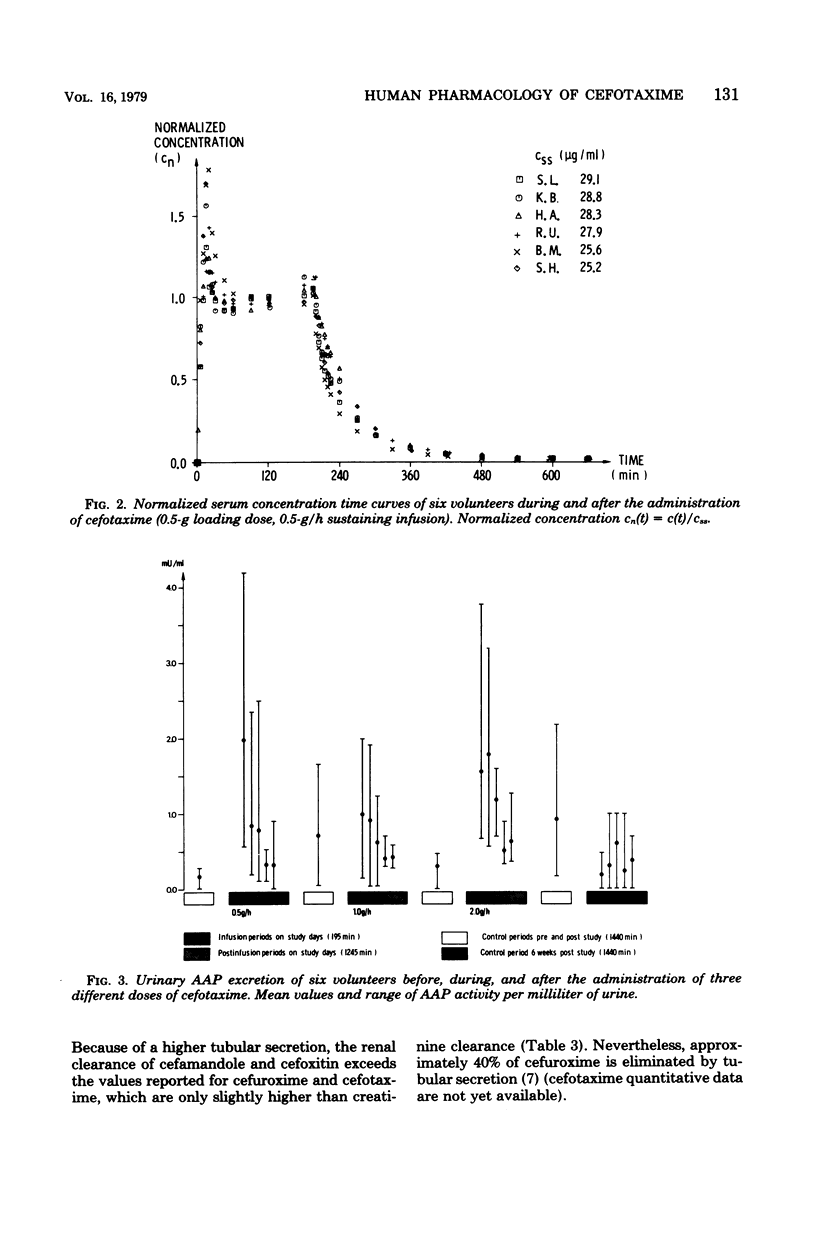
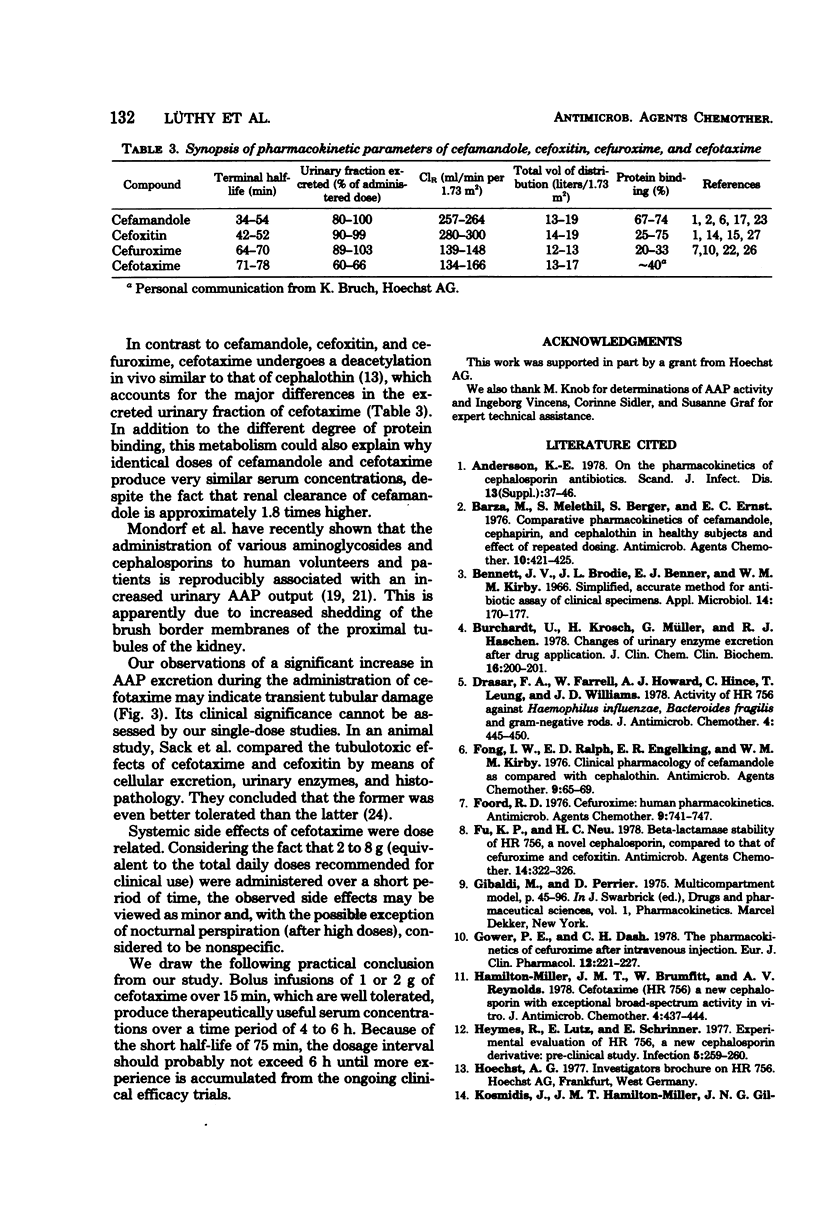
Cefotaxime (HR 756) is a new semisynthetic parenteral cephalosporin with exceptional activity against gram-negative organisms and considerable stability against their β-lactamases. To study its pharmacokinetic properties, 0.5-, 1-, and 2-g doses were administered to each of six volunteers intravenously over 15 min, followed by a sustaining infusions of 0.5, 1, and 2 g/h, respectively, for 3 consecutive hours. The loading doses produced mean peak levels of 41, 93, and 160 μg/ml, and mean steady-state serum concentrations were 27, 64, and 138 μg/ml, respectively. The mean terminal half-life was 75 ± 7 min. The total volume of distribution averaged 0.22 ± 0.03 liters/kg of body weight. Total body and renal clearances were 232 ± 30 and 145 ± 24 ml/min per 1.73 m2, respectively; 63 ± 9% of the administered dose was excreted through the kidneys in 24 h. To determine the effect of cefotaxime on the renal tubules, urinary alanine aminopeptidase excretion was measured before, during, and after the infusions. It remained within the normal range in all instances; however, 48 ± 14% of the total daily alanine aminopeptidase output was recovered during the infusion period. Side effects were dose related and included fatigue, loose stools, and night sweats. No significant changes in hematology, serum chemistry, or urinalysis were recorded.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barza M., Melethil S., Berger S., Ernst E. C. Comparative pharmacokinetics of cefamandole, cephapirin, and cephalothin in healthy subjects and effect of repeated dosing. Antimicrob Agents Chemother. 1976 Sep;10(3):421–425. doi: 10.1128/aac.10.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasar F. A., Farrell W., Howard A. J., Hince C., Leung T., Williams J. D. Activity of HR 756 against Haemophilus influenzae, Bacteroides fragilis and Gram-negative rods. J Antimicrob Chemother. 1978 Sep;4(5):445–450. doi: 10.1093/jac/4.5.445. [DOI] [PubMed] [Google Scholar]
- Fong I. W., Ralph E. D., Engelking E. R., Kirby W. M. Clinical pharmacology of cefamandole as compared with cephalothin. Antimicrob Agents Chemother. 1976 Jan;9(1):65–69. doi: 10.1128/aac.9.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foord R. D. Cefuroxime: human pharmacokinetics.. Antimicrob Agents Chemother. 1976 May;9(5):741–747. doi: 10.1128/aac.9.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. P., Neu H. C. beta-lactamase stability of HR 756, a novel cephalosporin, compared to that of cefuroxime and cefoxitin. Antimicrob Agents Chemother. 1978 Sep;14(3):322–326. doi: 10.1128/aac.14.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower P. E., Dash C. H. The pharmacokinetics of cefuroxime after intravenous injection. Eur J Clin Pharmacol. 1977 Nov 14;12(3):221–227. doi: 10.1007/BF00609865. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M., Brumfitt W., Reynolds A. V. Cefotoxime (HR 756) a new cephalosporin with exceptional broad-spectrum activity in vitro. J Antimicrob Chemother. 1978 Sep;4(5):437–444. doi: 10.1093/jac/4.5.437. [DOI] [PubMed] [Google Scholar]
- Heymès R., Lutz A., Schrinner E. Experimental evaluation of HR756, a new cephalosporin derivative: pre-clinical study. Infection. 1977;5(4):259–260. doi: 10.1007/BF01640793. [DOI] [PubMed] [Google Scholar]
- Kosmidis J., Hamilton-Miller J. M., Gilchrist J. N., Kerry D. W., Brumfitt W. Cefoxitin, a new semi-synthetic cephamycin: an in-vitro and in-vivo comparison with cephalothin. Br Med J. 1973 Dec 15;4(5893):653–655. doi: 10.1136/bmj.4.5893.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers B. R., Ribner B., Yancovitz S., Hirschman S. Z. Pharmacological studies with cefamandole in human volunteers. Antimicrob Agents Chemother. 1976 Jan;9(1):140–144. doi: 10.1128/aac.9.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondorf A. W., Breier J., Hendus J., Scherberich J. E., Mackenrodt G., Shah P. M., Stille W., Schoeppe W. Effect of aminoglycosides on proximal tubular membranes of the human kidney. Eur J Clin Pharmacol. 1978 May 17;13(2):133–142. doi: 10.1007/BF00609758. [DOI] [PubMed] [Google Scholar]
- Mondorf A. W., Breier J., Zegelman M., Müller H. Die Wirkung von Aminoglykosiden auf den proximalen Tubulus des Menschen. Adv Clin Pharmacol. 1978;15:101–110. [PubMed] [Google Scholar]
- Mondorf A. W. Die Bedeutung lokalisationsspezifischer Enzyme im Harn für toxikologische und klinisch-pharmakologische Untersuchungen. Arzneimittelforschung. 1973 Nov;23(11 Suppl):1634–1637. [PubMed] [Google Scholar]
- Mondorf A. W., Zegelman M., Klose J., Maske L., Scherberich J. E., Stefanescu T., Müller H., Schoeppe W. Effects of various cephalosporins on the proximal tubule of the human kidney. Eur J Clin Pharmacol. 1978 Jul 30;13(5):357–363. doi: 10.1007/BF00644609. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Sykes R. B., Ryan D. M., Foord R. D., Muggleton P. W. Cefuroxime - a new cephalosporin antibiotic. J Antibiot (Tokyo) 1976 Jan;29(1):29–37. doi: 10.7164/antibiotics.29.29. [DOI] [PubMed] [Google Scholar]
- Sack K., Lepére A., Schwieder G. Nierenverträglichkeit von Cephalosporinantibiotika: Cefoxitin und HR 756. Med Welt. 1978 Aug 11;29(32):1233–1236. [PubMed] [Google Scholar]
- Scherberich J. E., Falkenberg F. W., Mondorf A. W., Müller H., Pfleiderer G. Biochemical and immunological studies on isolated brush border membranes of human kidney cortex and their membrane surface proteins. Clin Chim Acta. 1974 Sep 16;55(2):179–197. doi: 10.1016/0009-8981(74)90294-0. [DOI] [PubMed] [Google Scholar]
- Simon C. Cefuroxim, ein neues beta-lactamase-stabiles Cephalosporin. Schweiz Med Wochenschr. 1978 Sep 9;108(36):1398–1403. [PubMed] [Google Scholar]
- Sonneville P. F., Kartodirdjo R. R., Skeggs H., Till A. E., Martin C. M. Comparative clinical pharmacology of intravenous cefoxitin and cephalothin. Eur J Clin Pharmacol. 1976 Mar 22;09(5-6):397–403. doi: 10.1007/BF00606555. [DOI] [PubMed] [Google Scholar]
- Stratford B. C. In-vitro activity of new cephalosporin (HR 756) and cefazolin. Lancet. 1978 Sep 2;2(8088):528–529. doi: 10.1016/s0140-6736(78)92255-9. [DOI] [PubMed] [Google Scholar]
- Vanhoff R., Butzler J. P., Yourassowsky E. In-vitro activity of new cephalosporin (HR 756) and cefazolin. Lancet. 1978 Jul 22;2(8082):209–210. doi: 10.1016/s0140-6736(78)91951-7. [DOI] [PubMed] [Google Scholar]


