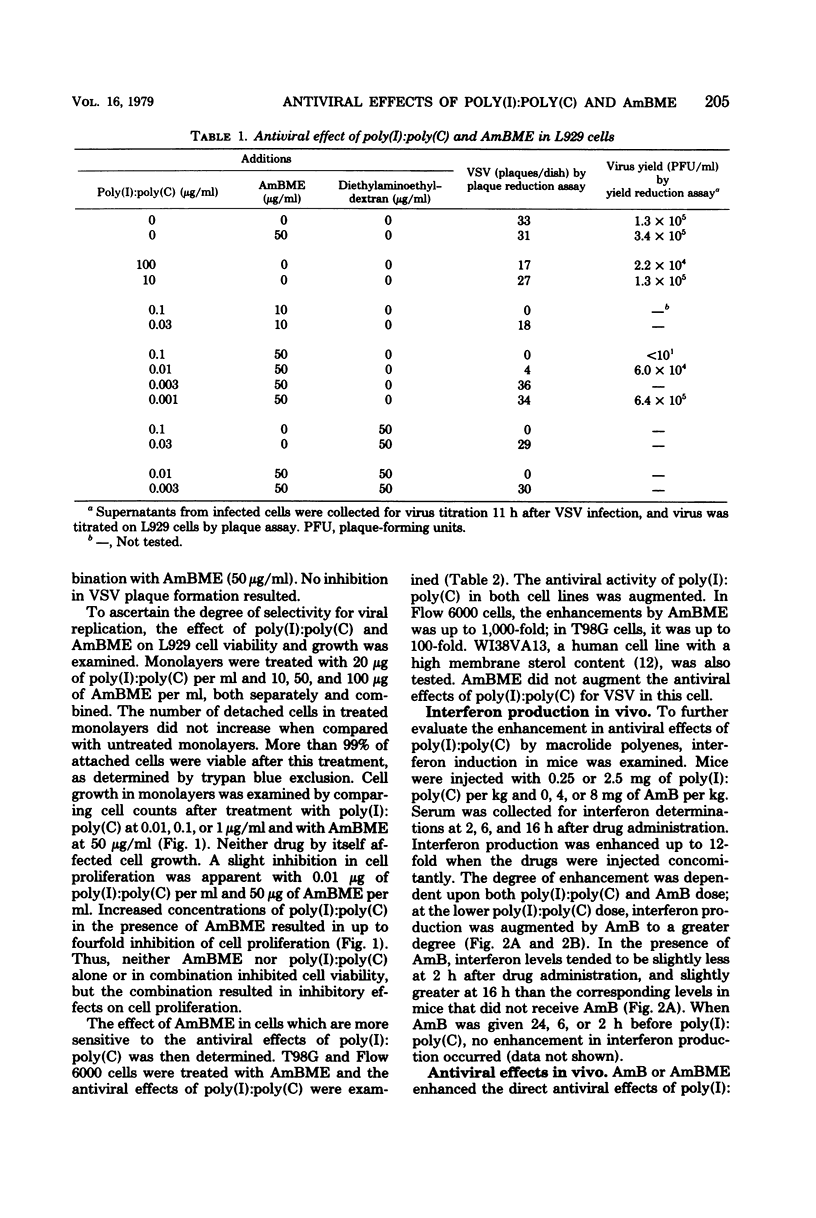
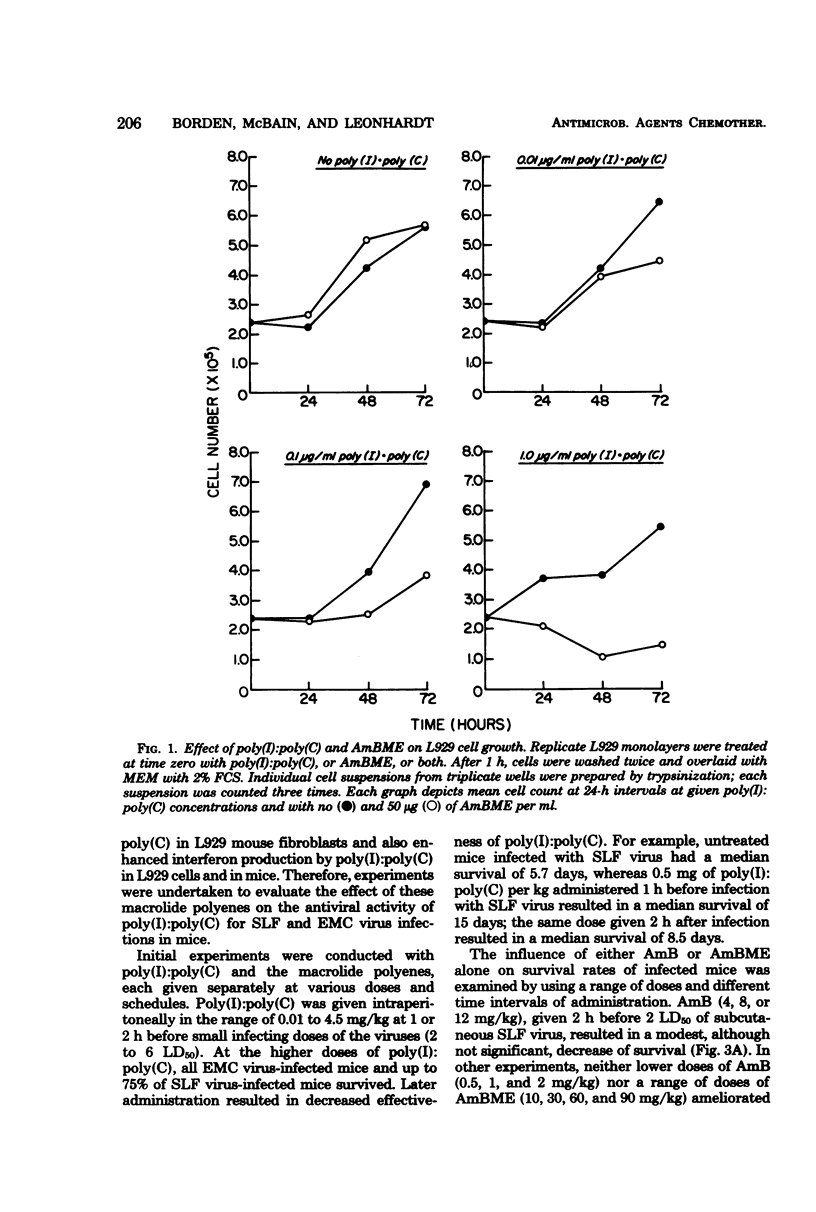
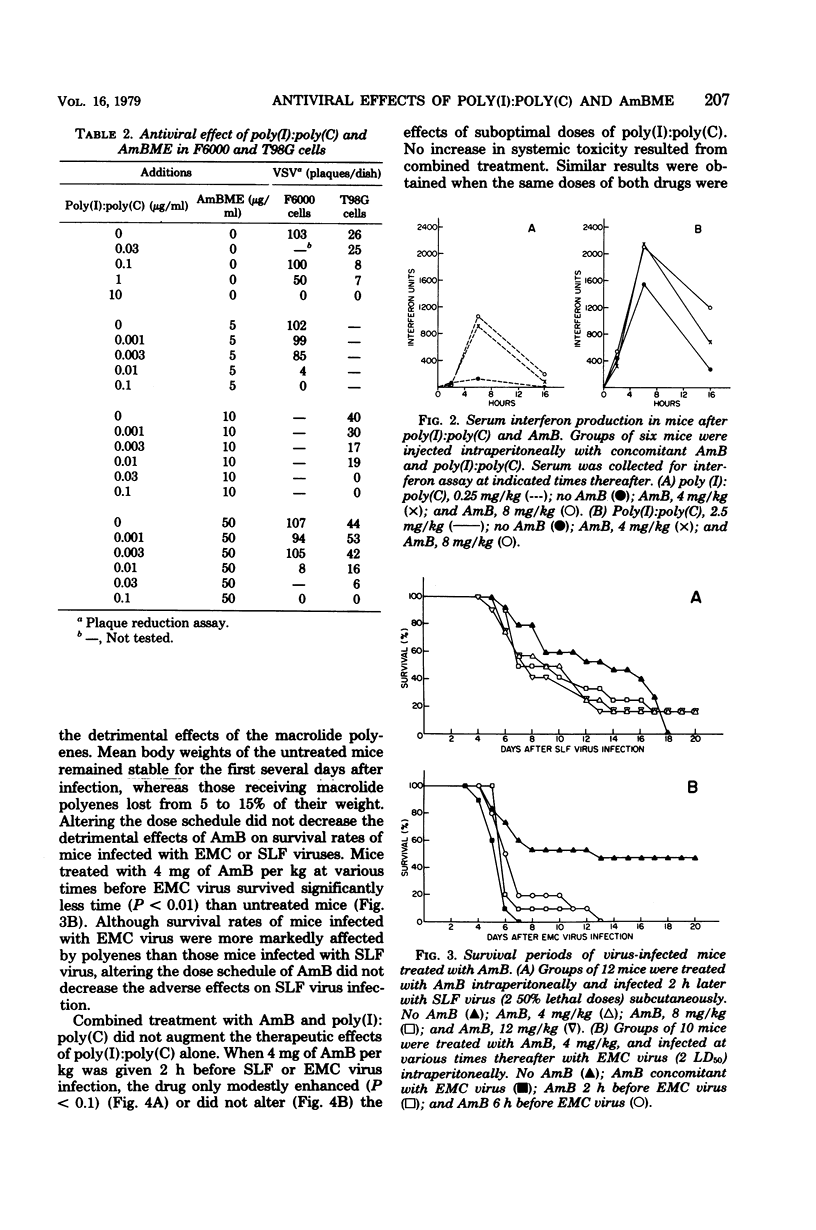
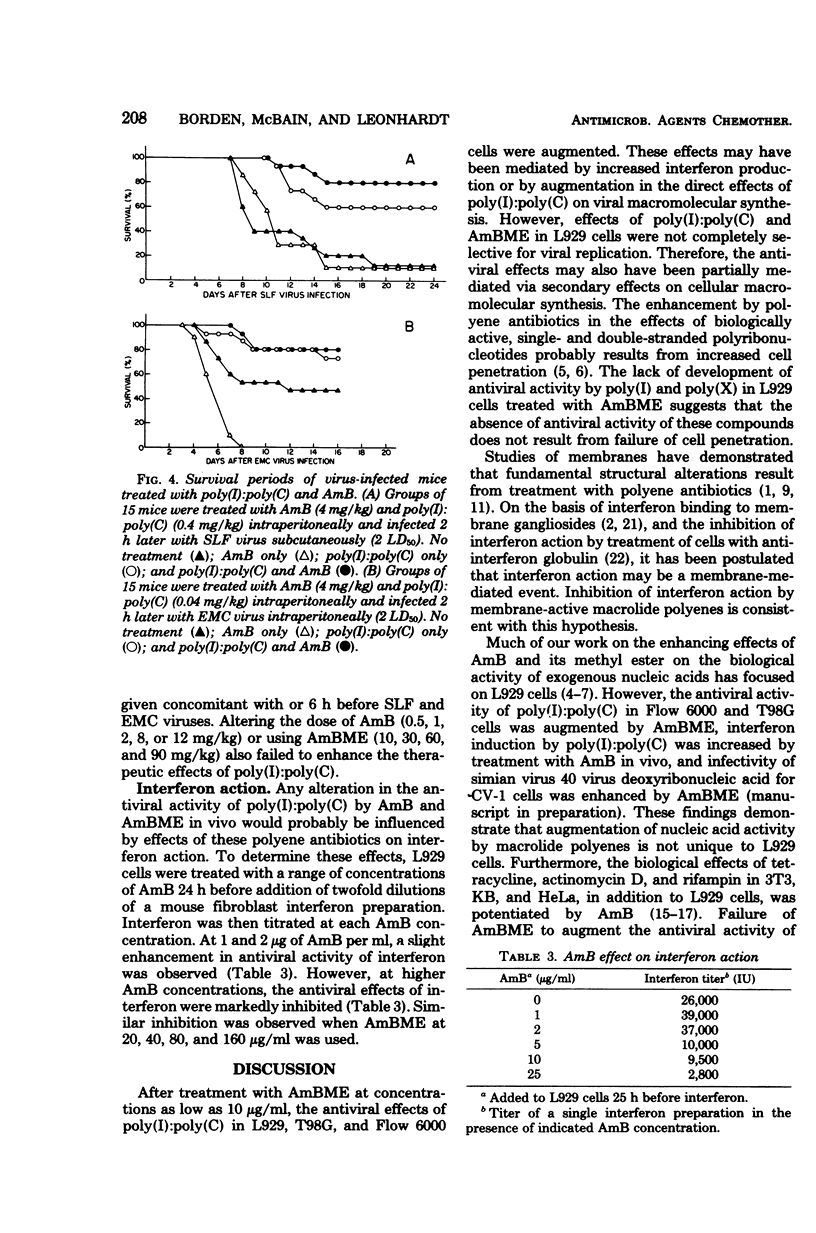
Abstract
Since the macrolide polyene antibiotics, amphotericin B (AmB) and its methyl ester (AmBME), augment interferon production by polyriboinosinic:polyribocytidylic acid [poly(I):poly(C)] in vitro, experiments were undertaken to determine how AmB and AmBME affect the antiviral activity of poly(I):poly(C) and interferon. AmBME increased the direct antiviral activity of poly(I):poly(C) 102-to 104-fold in L929, Flow 6000, and T98G cells. Viral replication, measured by either direct plaque formation or virus yield, was markedly reduced. Serum interferon levels in mice induced by poly(I):poly(C) were enhanced by concomitant treatment with AmB. However, the therapeutic effects of poly(I):poly(C) in encephalomyocarditis and Semliki Forest virus infections were not augmented by combined treatment with poly(I):poly(C) and AmB. In vitro, the antiviral effects of exogenous interferon were markedly inhibited by AmB and AmBME. This inhibition may have contributed to the adverse effects of the macrolide polyenes in encephalomyocarditis and Semliki Forest virus infections in vivo. These findings further substantiate the effectiveness of macrolide polyenes in augmenting cellular penetration of macromolecules. However, therapeutic application may be limited by the complex interactions which occur between compounds administered in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli T. E. On the anatomy of amphotericin B-cholesterol pores in lipid bilayer membranes. Kidney Int. 1973 Nov;4(5):337–345. doi: 10.1038/ki.1973.126. [DOI] [PubMed] [Google Scholar]
- Besancon F., Ankel H., Basu S. Specificity and reversibility of interferon ganglioside interaction. Nature. 1976 Feb 19;259(5544):576–578. doi: 10.1038/259576a0. [DOI] [PubMed] [Google Scholar]
- Blanke T. J., Little J. R., Shirley S. F., Lynch R. G. Augmentation of murine immune responses by amphotericin B. Cell Immunol. 1977 Sep;33(1):180–190. doi: 10.1016/0008-8749(77)90145-9. [DOI] [PubMed] [Google Scholar]
- Borden E. C., Booth B. W., Leonhardt P. H. Mechanistic studies of polyene enhancement of interferon production by polyriboinosinic-polyribocytidylic acid. Antimicrob Agents Chemother. 1978 Feb;13(2):159–164. doi: 10.1128/aac.13.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden E. C., Booth B. W., McBain J. A. Enhancement of infectivity of encephalomyocarditis virus RNA by amphotericin B methyl ester. J Gen Virol. 1979 Feb;42(2):297–303. doi: 10.1099/0022-1317-42-2-297. [DOI] [PubMed] [Google Scholar]
- Borden E. C., Leonhardt P. H. Aquanititave semimicro, semiautomated colorimetric assay for interferon. J Lab Clin Med. 1977 May;89(5):1036–1042. [PubMed] [Google Scholar]
- Borden E. C., Leonhardt P. H. Enhancement of rIn:rCn-induced interferon production by amphotericin B. Antimicrob Agents Chemother. 1976 Mar;9(3):551–553. doi: 10.1128/aac.9.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden E. C. Synergistic cytotoxicity for L929 cells of polyriboinosinic-polyribocytidylic acid and amphotericin B methyl ester. J Natl Cancer Inst. 1978 Jun;60(6):1309–1311. doi: 10.1093/jnci/60.6.1309. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Holz R. Aqueous pores created in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. Membranes. 1973;2:377–408. [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Fungal sterols and the mode of action of the polyene antibiotics. Adv Appl Microbiol. 1974;17(0):109–134. doi: 10.1016/s0065-2164(08)70556-2. [DOI] [PubMed] [Google Scholar]
- Howard B. V., Kritchevsky D. The lipids of normal diploid (WI-38) and SV40-transformed human cells. Int J Cancer. 1969 Jul 15;4(4):393–402. doi: 10.1002/ijc.2910040404. [DOI] [PubMed] [Google Scholar]
- Kinsky S. C. Antibiotic interaction with model membranes. Annu Rev Pharmacol. 1970;10:119–142. doi: 10.1146/annurev.pa.10.040170.001003. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Medoff G., Kobayashi G. S. Effects of amphotericin B on macrophages and their precursor cells. Antimicrob Agents Chemother. 1977 Jan;11(1):154–160. doi: 10.1128/aac.11.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G., Kwan C. N., Schlessinger D., Kobayashi G. S. Permeability control in animal cells by polyenes: a possibility. Antimicrob Agents Chemother. 1973 Mar;3(3):441–443. doi: 10.1128/aac.3.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G., Kwan C. N., Schlessinger D., Kobayashi G. S. Potentiation of rifampicin, rifampicin analogs, and tetracycline against animal cells by amphotericin B and polymyxin B. Cancer Res. 1973 Jun;33(6):1146–1149. [PubMed] [Google Scholar]
- Medoff G., Schlessinger D., Kobayashi G. S. Sensitivity of transformed and nontransformed cells to amphotericin B and several rifamycin derivatives. Cancer Res. 1974 Apr;34(4):823–826. [PubMed] [Google Scholar]
- Medoff G., Valeriote F., Lynch R. G., Schlessinger D., Kobayashi G. S. Synergistic effect of amphotericin B and 1,3-bis(2-chloroethyl)-1-nitrosourea against a transplantable AKR leukemia. Cancer Res. 1974 May;34(5):974–978. [PubMed] [Google Scholar]
- Morton B. E., Lardy H. A. Cellular oxidative phosphorylation. 3. Measurement in chemically modified cells. Biochemistry. 1967 Jan;6(1):57–61. doi: 10.1021/bi00853a011. [DOI] [PubMed] [Google Scholar]
- Presant C. A., Klahr C., Santala R. Amphotericin B induction of sensitivity to adriamycin, 1,3-bis (2-chloroethyl)-1-nitrosourea (BCNU) plus cyclophosphamide in human neoplasia. Ann Intern Med. 1977 Jan;86(1):47–51. doi: 10.7326/0003-4819-86-1-47. [DOI] [PubMed] [Google Scholar]
- Vengris V. E., Reynolds F. H., Jr, Hollenberg M. D., Pitha P. M. Interferon action: role of membrane gangliosides. Virology. 1976 Jul 15;72(2):486–493. doi: 10.1016/0042-6822(76)90177-x. [DOI] [PubMed] [Google Scholar]
- Vengris V. E., Stollar B. D., Pitha P. M. Interferon externalization by producing cell before induction of antiviral state. Virology. 1975 Jun;65(2):410–417. doi: 10.1016/0042-6822(75)90046-x. [DOI] [PubMed] [Google Scholar]


