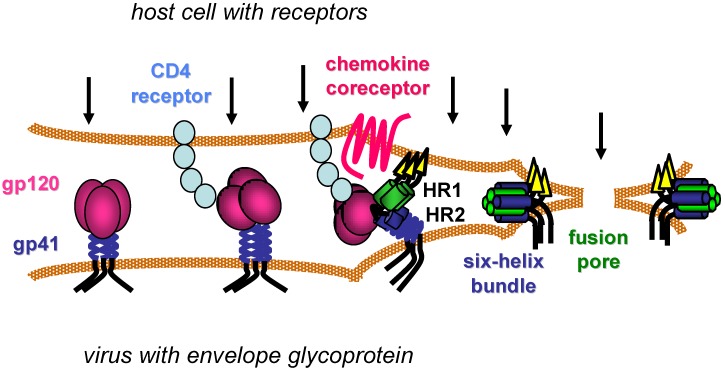
Figure 1.
Model of HIV entry. CD4 receptors and chemokine coreceptors are shown on the host cell. The gp120 surface subunit and gp41 transmembrane subunit of the HIV envelope glycoprotein are shown on viral membrane (envelope). After gp120 binds to CD4, the envelope glycoprotein undergoes conformational changes that facilitate gp120 interaction with the chemokine co-receptor. Additional conformational changes in the gp41 transmembrane subunit transiently expose two heptad-repeat domains (HR1 and HR2) that subsequently self-assemble to form a six-helix bundle structure. Formation of several gp41 six-helix bundles bring the host and viral membranes together for fusion, while several six‑helix bundles likely coalesce to form a fusion pore that allows the viral core to pass into the host cell cytoplasm. Arrows indicate potential steps in the entry process for inhibition.