Abstract
Background
Dioscorea bulbifera is an African medicinal plant used to treat microbial infections. In the present study, the methanol extract, fractions (DBB1 and DBB2) and six compounds isolated from the bulbils of D. bulbifera, namely bafoudiosbulbins A (1), B (2), C (3), F (4), G (5) and 2,7-dihydroxy-4-methoxyphenanthrene (6), were tested for their antimicrobial activities against Mycobacteria and Gram-negative bacteria involving multidrug resistant (MDR) phenotypes expressing active efflux pumps.
Methods
The microplate alamar blue assay (MABA) and the broth microdilution methods were used to determine the minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) of the above samples.
Results
The results of the MIC determinations indicated that when tested alone, the crude extract, fractions DBB1 and DBB2 as well as compounds 2 to 5 were able to prevent the growth of all the fifteen studied microorganisms, within the concentration range of 8 to 256 μg/mL. The lowest MIC value for the methanol extract and fractions (16 μg/mL) was obtained with DBB1 and DBB2 on E, coli AG100A and DBB2 on Mycobacterium tuberculosis MTCS2. The lowest value for individual compounds (8 μg/mL) was recorded with compound 3 on M. smegmatis and M. tuberculosis ATCC and MTCS2 strains respectively. The activity of the samples on many MDR bacteria such as Enterobacter aerogenes EA289, CM64, Klebsiella pneumoniae KP63 and Pseudomonas aeruginosa PA124 was better than that of chloramphenicol. When tested in the presence of the efflux pump inhibitor against MDR Gram-negative bacteria, the activity of most of the samples increased. MBC values not greater than 512 μg/mL were recorded on all studied microorganisms with fraction DBB2 and compounds 2 to 5.
Conclusions
The overall results of the present investigation provided evidence that the crude extract D. bulbifera as well as some of the compounds and mostly compounds 3 could be considered as potential antimicrobial drugs to fight against MDR bacteria.
Keywords: Diterpenoids, Antimycobacterial, Antibacterial, Dioscorea bulbifera, Dioscoreaceae
Background
The continuous emergence of Gram-negative MDR bacteria drastically reduces the efficacy of our antibiotic armory and, consequently, increases the frequency of therapeutic failure [1]. On the other hand, the World Health Organization (WHO) estimates that there are nine million cases of tuberculosis (TB) currently, with 1.3 million reported deaths every year, 55 and 30% of the TB burden being shared by Asian and African countries respectively [2]. Approximately 60% of world’s population still relies on medicinal plants for their primary healthcare. Medicinal plants have been used as a source of remedies since ancient times in Africa. Dioscorea bulbifera L. var sativa (Dioscoreaceae) is an African medicinal plant used to treat microbial infections and pig cysticercosis by the native people of western highlands of Cameroon. The plant is also used as a folk remedy to treat conjunctivitis, diarrhea and dysentery, among other ailments [3]. Previous phytochemical study on this medicinal plant led to the isolation and structural elucidation of seven new clerodane diterpenoids namely Bafoudiosbulbins A-G [4-6]. Furthermore, the extracts and Bafoudiosbulbins A and B were shown to possess anti-Salmonella activity [4]. In the present study, the bioguided fractionation was undertaken in order to deeply evaluate the antimicrobial activity of D. bulbifera.
Methods
Plant material
The bulbils of D. bulbifera L. var sativa were collected in Bafou village near Dschang (West region of Cameroon) in February 2007. The plant was identified at the National Herbarium (Yaoundé, Cameroon) where a voucher specimen was deposited under the reference number 22211/SRF/CAM.
Extraction and isolation
The air-dried bulbils of D. bulbifera L. var sativa (2 kg) were pulverized and extracted three times (each time for 24 h) with MeOH. The methanol extract was concentrated under reduced pressure to yield a dark residue (DBB; 90 g). Part of this (80 g) was suspended in water (150 mL) and submitted to successive partition with Ethyl acetate (EtOAc) and n-butanol. The EtOAc and n-butanol layers were concentrated to dryness under reduced pressure to afford 35 g and 23 g of extracts respectively. The n-butanol extract showed no antimicrobial activity contrary to the EtOAc extract (Tables 1 and 2). Part of the EtOAc extract (DBB1; 28 g) was subjected to column chromatography over silica gel 60 Merck [0.040–0.063 mm; 56 g] using hexane-EtOAc with increasing polarity as eluents to yield five main sub-fractions named A-E. They were then screened for their antimicrobial activities and sub-Fraction C (DBB2) eluted with Hexane-EtOAc 4–6 to 3–7 was the only active sample. DBB2 (m = 4.8 g) was then fractionated and purified using column chromatography over silica gel and sephadex LH-20 to yield compound 1 {White needles; [α]D21 = − 64.6 (c = 0.025, DMSO); 60 mg; Rf = 0.78, CH2Cl2-MeOH 95–5; C21H22O8m/z 403 [M+H]+; m.p. = 252-253°C}, compound 2 {White needles; [α]D21 = +52 (c = 0.010, pyridine); 71 mg; Rf = 0.44, CH2Cl2-MeOH 95–5; C20H20O8, m/z 387 [M−H]−; m.p. = 312-313°C}, compound 3 {White gum; [α]D21 = +56.2° (c = 0.8, CH2Cl2); 32 mg; Rf = 0.37, CH2Cl2-MeOH 95–5; C21H22O7, 358 [M−CO]+}, compound 4 {White needles; [α]D21 = − 5o (c = 0.6, CH2Cl2); 58 mg; Rf = 0.56, CH2Cl2-MeOH 95–5; C21H24O8; m/z 439 [M+Cl]; m.p. = 265-266°C}, compound 5 {White crystals; [α]D20 = − 46o (c = 0.9, CD3OD); 64 mg; Rf = 0.41, CH2Cl2-MeOH 95–5; C23H26O10; m/z 485 [M+Na]+; m.p. = 199-200°C}, compound 6 {Yellow amorphous powder; 28 mg; Rf = 0.48, CH2Cl2-MeOH 95–5; C15H12O3, m/z 241 [M+H]+}.
Table 1.
MICs of the extract, fractions, compounds from Dioscorea bulbifera and chloramphenicol on documented strains and clinical MDR isolates
|
Samplesa |
Microorganisms and MIC(μg/mL)without and in the presence of PAßN(in parenthesis)b |
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
E.coli |
E.aerogenes |
K.pneumoniae |
P.aeruginosa |
M.smegmatis |
M.tuberculosis |
|||||||||
| ATCC8739 | AG100A | AG102 | ATCC13048 | EA289 | EA-CM64 | ATCC11296 | KP55 | KP63 | PA01 | PA124 | ATCC 700084 | ATCC 27294 | MTCS1 | MTCS2 | |
| DBB |
64 (64) |
64 (32) |
128 (128) |
64 (64) |
256 (128) |
256 (256) |
64 (64) |
128 (128) |
128 (128) |
128 (128) |
128 (128) |
64 |
64 |
256 |
64 |
| DBB1 |
32 (32) |
16 (8) |
128 (128) |
32 (32) |
64 (64) |
64 (64) |
32 (32) |
64 (64) |
64 (64) |
64 (64) |
64 (64) |
3x2 |
64 |
256 |
32 |
| DBB2 |
64 (64) |
16 (8) |
64 (64) |
32 (16) |
64 (64) |
64 (64) |
64 (64) |
64 (64) |
32 (16) |
32 (16) |
64 (64) |
32 |
32 |
64 |
16 |
|
1 |
256 (64) |
128 (32) |
- |
256 (128) |
- (256) |
- |
256 (64) |
512 (128) |
- (256) |
- (512) |
- |
- |
nd |
nd |
nd |
|
2 |
64 (32) |
64 (64) |
64 (32) |
128 (64) |
64 (64) |
64 (64) |
64 (32) |
128 (64) |
64 (32) |
128 (64) |
128 (64) |
32 |
32 |
32 |
16 |
|
3 |
64 (16) |
16 (8) |
64 (16) |
64 (16) |
64 (16) |
64 (16) |
64 (32) |
64 (16) |
64 (32) |
128 (32) |
128 (32) |
8 |
8 |
32 |
8 |
|
4 |
64 (64) |
64 (32) |
64 (64) |
128 (32) |
64 (32) |
128 (64) |
64 (64) |
64 (64) |
64 (32) |
128 (64) |
128 (64) |
32 |
64 |
64 |
32 |
|
5 |
64 (64) |
64 (32) |
128 (64) |
128 (64) |
128 (32) |
256 (64) |
128 (128) |
128 (64) |
256 (64) |
256 (64) |
128 (32) |
64 |
32 |
64 |
32 |
|
6 |
256 (256) |
256 (256) |
- (512) |
- (512) |
- (256) |
- |
256 (128) |
- (256) |
- (256) |
- (256) |
- (512) |
- |
nd |
nd |
nd |
| RA | 4 (1) | 1 (0.5) | 16 (1) | 4 (1) | 512 (128) | 256 (8) | 2 (2) | 32 (4) | 512 (128) | 128 (8) | 256 (8) | 0.5 | 0.5 | 64 | 2 |
aTested samples [DBB: methanol extract from the bulbils of D. bulbifera; bafoudiosbulbins A (1), B (2), C (3), F (4), G (5), 2,7-dihydroxy-4-methoxyphenanthrene (6); EtOAc fraction (DBB1) and EtOAc sub-fraction C (DBB2); RA : reference antibiotics were chloramphenicol for bacteria, ciprofloxacin for M. smegmatis, isoniazid for M.tuberculosis; (−): MIC > 512 μg/mL; bThe drugs and compounds were tested in the absence or in the presence (values in braket) of PAßN at a final concentration of 20 μg/mL, as described previously [7]. At this concentration, no intrinsic effect against the various bacterial strains (included as internal controls in each assay without antibiotic) was observed.
Table 2.
MBCs of the extract, fractions, compounds from Dioscorea bulbifera and chloramphenicol on documented strains and clinical MDR isolates
|
Samples |
Microorganisms and MBC(μg/mL)without and in the presence of PAßN(in parenthesis)* |
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
E.coli |
E.aerogenes |
K.pneumoniae |
P.aeruginosa |
M.smegmatis |
M.tuberculosis |
|||||||||
| ATCC8739 | AG100A | AG102 | ATCC13048 | EA289 | EA-CM64 | ATCC11296 | KP55 | KP63 | PA01 | PA124 | ATCC 700084 | ATCC 27294 | MTCS1 | MTCS2 | |
| DBB |
128 (128) |
128 (64) |
256 (256) |
128 (128) |
512 (512) |
- (512) |
256 (256) |
256 (256) |
256 (256) |
256 (256) |
256 (256) |
128 |
128 |
- |
128 |
| DBB1 |
128 (64) |
32 (16) |
512 (256) |
64 (64) |
128 (128) |
256 (128) |
64 (64) |
128 (128) |
256 (128) |
128 (128) |
128 (128) |
64 |
128 |
- |
64 |
| DBB2 |
256 (128) |
32 (16) |
256 (128) |
64 (32) |
128 (128) |
256 (128) |
128 (128) |
128 (128) |
64 (64) |
64 (64) |
256 (128) |
128 |
128 |
128 |
64 |
|
1 |
- (256) |
256 (128) |
nd |
- (256) |
nd |
nd |
- (128) |
- (256) |
nd |
nd |
nd |
- |
nd |
nd |
nd |
|
2 |
128 (128) |
128 (64) |
128 (128) |
256 (128) |
128 (128) |
128 (128) |
128 (128) |
256 (128) |
128 (128) |
512 (128) |
256 (128) |
64 |
128 |
128 |
64 |
|
3 |
128 (32) |
128 (32) |
256 (32) |
128 (32) |
256 (32) |
128 (32) |
128 (64) |
128 (64) |
256 (64) |
256 (64) |
256 (64) |
8 |
16 |
64 |
16 |
|
4 |
128 (128) |
128 (64) |
256 (128) |
256 (128) |
128 (64) |
512 (128) |
256 (128) |
256 (128) |
128 (128) |
256 (128) |
256 (128) |
64 |
128 |
128 |
128 |
|
5 |
128 (128) |
128 (128) |
256 (128) |
256 (128) |
256 (128) |
- (128) |
512 (256) |
512 (128) |
- (128) |
512 (256) |
512 (128) |
256 |
128 |
256 |
128 |
|
6 |
- |
- (512) |
- |
nd (−) |
nd (256) |
nd |
- (512) |
nd (256) |
nd (256) |
nd (256) |
nd (512) |
- |
nd |
nd |
nd |
| RA | 8 (2) | 4 (1) | 32 (2) | 8 (2) | - (256) | 512 (16) | 4 (4) | 64 (8) | - (256) | 256 (16) | 512 (16) | 1 | 1 | 128 | 4 |
aTested samples [DBB: methanol extract from the bulbils of D. bulbifera; bafoudiosbulbins A (1), B (2), C (3), F (4), G (5), 2,7-dihydroxy-4-methoxyphenandhrene (6); EtOAc fraction (DBB1) and EtOAc sub-fraction C (DBB2); RA : reference andibiotics were chloramphenicol for bacteria, ciprofloxacin for M. smegmatis, isoniazid for M.tuberculosis; (−): MIC > 512 μg/mL; bThe drugs and compounds were tested in the absence or in the presence (values in braket) of PAßN at a final concendration of 20 μg/mL, as described previously [7]. At this concendration, no indrinsic effect against the various bacterial strains (included as indernal condrols in each assay without andibiotic) was observed; (nd): not determined.
General procedure
Melting points were determined using the GallenkampMelting Point Apparatus. Optical rotations were measured on a Perkin–Elmer 241 Polarimeter, IR spectra were measured as a film on a KBr pellet using a FTIR-8400S Shimadzu spectrometer. ESIMS was carried out on a Hewlett Packard HP-1100 series LC–MSD system and on the mass spectrometer Brucker FTMS4.7T, BIOAPEXII. 1H NMR spectra were recorded in deuterated solvents (DMSO and C5H5N) on a on a Varian Mercury Plus Spectrometer at 400 MHz while 13C NMR spectra were recorded in the same solvents and the same apparatus at 100 MHz. All chemical shifts (δ) are given in ppm units with reference to tetramethylsilane (TMS) as internal standard and the coupling constants (J) are in Hz. Column chromatography was performed using silica gel 60 Merck (0.040–0.063 mm) and sephadex LH-20. TLC were carried out on precoated Kieselgel 60 F254 (Merck) plates developed with hexane:AcOEt (7:3) and AcOEt:MeOH (98:2). TLC plates were viewed with an ultraviolet lamp MULTIBAND UV-254/365 nm for fluorescent spots. They were also visualized by spraying with 50% H2SO4 and heating for 10 min at 110°C.
Antimicrobial assays
Chemicals for antimicrobial assay
Chloramphenicol ≥ 98% (Sigma-Aldrich, St. Quentin Fallavier, France) was used as reference antibiotics (RA) against Gram-negative bacteria. p-Iodonitrotetrazolium chloride ≥ 97% (INT, Sigma-Aldrich) was used as microbial growth indicator [8,9]. Ciprofloxacin ≥ 98% and isoniazid ≥ 99% (INH) (Sigma) were used as reference antibiotics (RA) for M. smegmatis and M. tuberculosis respectively. Phenylalanine arginine β-naphthylamide ≥ 98% (PAßN, Sigma-Aldrich) was used as microbial growth indicator and efflux pumps inhibitor.
Microbial strains and culture media
The studied microorganisms included strains of Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterobacter aerogenes, Escherichia coli, four Mycobacteria namely M. smegmatis, drug-susceptible strain of M. tuberculosis H37Rv obtained from the American Type Culture Collection, and two clinical strains of M. tuberculosis MTCS1, MTCS2. M. smegmatis was cultured on Middlebrook 7H11 agar and allowed to grow for 24 h. M. tuberculosis was plated on Löwenstein–Jensen medium and allowed to grow for 3–4 weeks at 37°C. Middlebrook 7H9 broth was used to determine the MIC and MBC values of the test samples on M. smegmatis and M. tuberculosis. Nutrient agar was used for the activation of Gram-negative bacteria [10]. The clinical features of the Gram-negative bacteria are available as Additional file 1.
INT colorimetric assay for MIC and MBC determinations
The MIC determinations on M. smegmatis and Gram-negative bacteria were conducted using rapid INT colorimetric assay according to described methods [8,9] with some modifications. The test samples and RA were first of all dissolved in DMSO/Mueller Hinton Broth (MHB) or DMSO/7H9 broth. The final concentration of DMSO was lower than 2.5% and does not affect the microbial growth [11]. The solution obtained was then added to 7H9 broth (M. smegmatis) or Mueller Hinton Broth (Gram-negative organisms), and serially diluted two fold (in a 96- wells microplate). One hundred microlitre (100 μL) of inoculum 1.5 x 106 CFU/mL prepared in appropriate broth was then added [12]. The plates were covered with a sterile plate sealer, then agitated to mix the contents of the wells using a plate shaker and incubated at 37°C for 18 h. The assay was repeated thrice. Wells containing adequate broth, 100 μL of inoculum and DMSO to a final concentration of 2.5% served as negative control. The MIC of samples was detected after 18 h incubation at 37°C, following addition (40 μL) of 0.2 mg/mL p-iodonitrotetrazolium chloride (INT) and incubation at 37°C for 30 min. Viable bacteria reduced the yellow dye to a pink. MIC was defined as the sample concentration that prevented the color change of the medium and exhibited complete inhibition of microbial growth [8,9]. The MBC was determined by adding 50 μL aliquots of the preparations, which did not show any growth after incubation during MIC assays, to 150 μL of adequate broth. These preparations were incubated at 37°C for 48 h. The MBC was regarded as the lowest concentration of extract, which did not produce a color change after addition of INT as mentioned above [11,13].
Samples were also tested in the presence of PAßN at 30 μg/mL final concentration [7] and the MIC was determined as above.
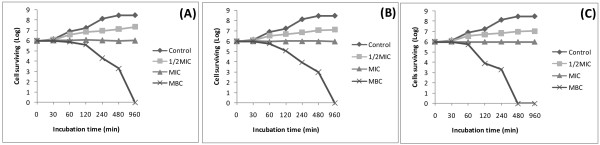
Time-kill dynamic curves against E. coli ATCC8739
Time-kill dynamic assay was performed using broth microdilution method as previously described [14] with minor modifications. The final concentration of suspension of the E. coli ATCC8739 strain was adjusted to 106 CFU/mL. The crude extract, fraction DBB2 and compound 3 were used in the time-kill dynamic experiment. Cells treated with concentrations corresponding to ½ MIC; MIC and MBC of each sample were incubated at 37°C for 0, 30, 60, 120, 240, 480, and 960 min. The final concentration of DMSO was 2.5%. A control sample was made using DMSO 2.5% and the inoculum. At each incubation time point, liquids (50 μl) were removed from the test solution for ten-fold serial dilution. Thereafter, a 25 μl liquid from each dilution was spread on the surface of the MHA plates and incubated at 37°C for 24 h, and the number of CFU/mL was counted. Experiments were carried out in triplicate. Time-kill curves were constructed by plotting the number of CFU/mL against time (min).
Microplate Alamar Blue assay against M. tuberculosis
The activity of all samples against M. tuberculosis strains was tested using the MABA [15]. Briefly, each of the above M. tuberculosis strains was cultured at 37°C in Middlebrook 7H9 broth supplemented with 0.2% glycerol and 10% Oleic Acid–Albumin–Dextrose–Catalase (Sigma) until logarithmic growth was reached. About 6x106 CFU/mL inoculum of M. tuberculosis was then added to the two fold serially diluted samples. The final concentration of DMSO in all assays was 2.5% or less and this dilution also served as solvent control. The samples were assayed in triplicate. All tests were carried out in sterile flat bottom 96-well microplates. Each microplate was incubated for 5 days at 37°C in a sealed plastic CO2-permeable bag. After 5 days of incubation, 32 μL of a mixture of freshly prepared Alamar Blue solution and 20% sterile Tween-80 (Sigma) 1:1 v/v were added to one growth-control well. The microplates were incubated again at 37°C for 24 h. If a color shift from blue to pink was observed in the growth-control sample, 32 μL of alamar blue solution was added to each of the remaining wells, and the microplate was further incubated for 24 h. A well-defined pink color was interpreted as positive bacterial growth, whereas a blue color indicated an absence of growth. The MIC corresponded to the greatest dilution of sample extract in which the color shift from blue to pink was not observed.
Samples with recorded MIC values following MABA were assayed for their mycobactericidal effect [15]. Briefly, 5 μL of the undeveloped mycobacterial suspensions were transferred from the former to a new microplate that contained 195 μL of fresh culture medium per well. Three wells were inoculated with 100 μL of fresh inoculum as for MABA and three more wells were incubated with 200 μL of culture medium only, as negative controls. The microplates were incubated and developed with alamar blue as for MABA. The MBC corresponded to the minimum sample concentration that did not cause a color shift in cultures re-incubated in fresh medium.
Results and discussion
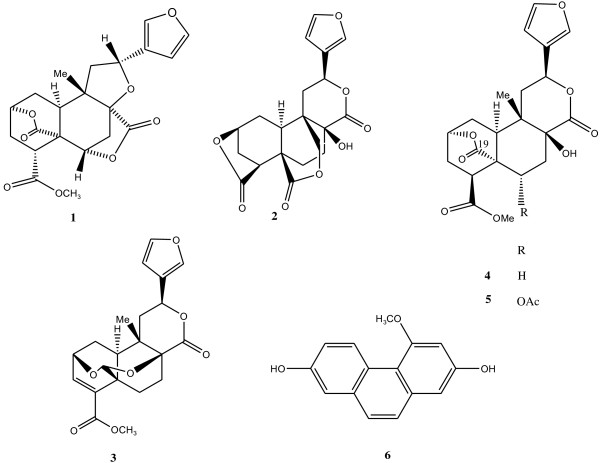
The structural elucidation of the isolated compounds was achieved using physical and spectroscopic techniques as described in our research group. They were identified as diterpenoids bafoudiosbulbins A (1), B (2) [4], C (3) [5], F (4), G (5) [6] and 2,7-dihydroxy-4-methoxyphenanthrene (6) [16]. Their chemical structures are illustrated in Figure 1. These compounds together with the crude extract and fractions were tested for their antimicrobial activities and the results are reported in Tables 1 and 2.
Figure 1.
Chemical structures of the compounds isolated from the bulbils of Dioscorea bulbifera.
The results of the MIC determinations (Table 1) indicated that when tested alone, the methanol crude extract, fractions DBB1 and DBB2 as well as compounds 2 to 5 were able to prevent the growth of all the fifteen studied microorganisms, including mycobacteria and Gram-negative bacteria, within the concentration range of 8 to 256 μg/mL. Compounds 1 and 6 showed selective activities, their inhibitory effects being noted respectively on 5/15 (33.3%) and 3/15 (20%) of the studied microorganisms. The lowest MIC value for the methanol extract and fractions (16 μg/mL) was obtained with DBB1 and DBB2 on E. coli AG100A and DBB2 on M. tuberculosis MTCS2. The lowest value for individual compounds (8 μg/mL) was recorded with compounds 3 on M. smegmatis and M. tuberculosis ATCC and MTCS2 strains. The activity of the samples on many MDR bacteria such as E. aerogenes EA289, CM64, K. pneumoniae KP63 and P. aeruginosa PA124 was better than that of chloramphenicol. When tested in the presence of the efflux pump inhibitor against Gram-negative bacteria expression active efflux (Table 1), the activity of most of the samples increased, the lowest MIC values obtained being 8 μg/mL for DBB1 and 2, compound 3 on E. coli AG100A. Results of MBC determinations (Table 2) also showed good activities for most of the tested samples. When tested in the absence of PAßN, MBC values not greater than 512 μg/mL were recorded on all studied microorganisms with fraction DBB2, compounds 2 to 5, on 13/15 (86.7%) and 14/15 (93.3%) of the studied organisms respectively the crude extract and fraction DBB1. As previously observed with MIC values, compounds 1 and 6 showed poor activities in the MBC test. Similarly to MICs data, the MBC values of the samples decrease when they were associated with PAßN. Also, it can be noted (Figure 1) that significant reduction of the bacterial population is observed with the crude extract, fraction DBB2 and compound 3 at a concentration corresponding to their MBC values.
In the present work, broad spectrum of antimicrobial activities was recorded with the crude extract, fractions and compounds from D. bulbifera. Phytochemicals are routinely classified as antimicrobials on the basis of susceptibility tests that produce MIC in the range of 100 to 1000 mg/mL [17]. Activity is considered to be significant if MIC values are below 100 μg/mL for crude extract and moderate when 100<MIC<625 μg/mL [18,19]. Therefore, the activity recorded with the crude extract on the ATCC strain of E. coli, E. aerogenes, K. pneumoniae, M. smegmatis and M. tuberculosis as well as E. coli AG100A and M. tuberculosis MTCS2, can be considered as important. Similarly, the activity recorded with the two studied fractions from D. bulbifera on the majority of the studied microbial strains was also significant. The activity of compound 3 on three of the four tested mycobacterial strains can also be considered good. The MIC values obtained with compound 3 on MDR bacteria such as E. aerogenes EA289, CM64, K. pneumoniae KP63 and P. aeruginosa PA124 was lower than those of the reference drug, highlighting its good antibacterial potency.
Keen look of the MBC values of the tested samples, alone or in the presence of the efflux pumps inhibitor indicates that most of them are not more than fourfold their corresponding MICs. This proves that the killing effects of many tested samples could be expected on the sensitive strains [20]. This can also be confirmed by the reduction of cell survival at the MBC values of the crude extract, fraction DBB2 and compound 3 as observed in Figure 2. Enterobacteriaceae, including K. pneumoniae, E. aerogenes and E. coli as well as P. aeruginosa and M. tuberculosis have been classified as antimicrobial-resistant organisms of concern in healthcare facilities [21-25]. The good activities of the crude extract, fractions and compound 3 on most of the tested microorganisms belonging to MDR phenotypes such as E. coli AG102, P. aeruginosa PA124, E. aerogenes CM64, K. pneumoniae KP55 and KP63 as observed herein reinforces the hypothesis that D. bulbifera can be a potential source of antimicrobial drugs. It should be noted that active compounds from D. bulbifera are substrates of MDR bacteria efflux pumps, suggesting a possible use of an inhibitor in the fight against such strains. In this work, only compounds with inhibitory activity on M. smegmatis were tested on M. tuberculosis. However, it has been demonstrated that the sensitivity of M. tuberculosis is closer to that of M. smegmatis, a non pathogenic microorganism [26]. Therefore, this microorganism can be used for a preliminary study to select samples with potential activity against M. tuberculosis[26]. Hence, the results obtained herein are in accordance with such recommendation.
Figure 2.
Survival curve for E.coli ATCC8739 cells exposed to the crude extract (A),fraction DBB2 (B) and compound 3 (C). (Control): MHB medium in DMSO 2.5% + inoculum.
To the best of our knowledge, the antimicrobial activities of D. Bulbifera against MDR bacteria and mycobacteria strain as well as those of the isolated compounds is being reported for the first time. However the anti-salmonellal activity of compounds 1 and 2 was reported [4]. Also, a norditerpene, 8-epidiosbulbin E acetate, from Dioscorea bulbifera was found active against MDR Escherichia coli and Pseudomonas aeruginosa with MIC values of 200 and 400 μg/mL respectively [27]. The mechanism of the active compounds is still to be studied; nevertheless, membrane disruption could be suggested as one of the likely mechanisms of action of 1 to 5, the compounds belonging to the terpenoids [28].
Conclusion
The data reported herein are very important, taking in account the medical importance of the studied microorganisms. Hence, the overall results of the present investigation provided evidence that the crude extract D. bulbifera as well as some of the compounds, and mostly compounds 3 could be considered as potential antimicrobial drug.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
VK, RBT and ATM carried out the study; VK, RBT and ATM wrote the manuscript; VK, LAT, LB, JJMM and NL supervised the work. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Table S1. Gram-negative bacterial strains and features. The studied bacteria included reference ATCC strain of E. coli ATCC8739, E. aerogenes ATCC13048, K. pneumoniae ATCC12296 and P. aeruginosa PA01 as well as MDR strains E. coli AG100A and AG102, E. aerogenes EA-CM64 and EA289, K. pneumoniae Kp55 and Kp63, and P. aeruginosa PA124.
Contributor Information
Victor Kuete, Email: Kuetevictor@yahoo.fr.
Rémy BetrandTeponno, Email: rteponno@yahoo.fr.
Armelle Tsafack Mbaveng, Email: armkuete@yahoo.fr.
Léon Azefack Tapondjou, Email: tapondjou2001@yahoo.fr.
Jacobus J Marion Meyer, Email: marion.meyer@up.ac.za.
Luciano Barboni, Email: luciano.barboni@unicam.it.
Namrita Lall, Email: Namrita.Lall@up.ac.za.
Acknowledgements
Authors are thankful to the Cameroon National Herbarium (Yaounde) for the plant identification. Authors are thankful to the Cameroon National Herbarium (Yaounde) for the plant identification. Authors are also grateful to the International Foundation for Science (IFS-Grant F/4579-2) and Organization for the Prohibition of Chemical Weapons (OPCW) through IFS to VK and IFS-Grant F/3976-2 to LAT). Authors are also thankful to UMR-MD1 (Mediterranean University, Marseille, France) for providing some clinical bacteria.
References
- Rice LB. Unmet medical needs in antibacterial therapy. Biochem Pharmacol. 2006;71:991–995. doi: 10.1016/j.bcp.2005.09.018. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Tuberculosis, Fact sheet No. 104. World Health Organization, Geneva; 2010. [Google Scholar]
- Duke J. A, DuCellier JL: Handbook of Alternative Cash Crops. Boca Raton, CRC Press, Florida; 1993. [Google Scholar]
- Teponno RB, Tapondjou AL, Gatsing D, Djoukeng JD, Abou-Mansour E, Tabacchi R, Tane P, Stoekli-Evans H, Lontsi D, Park HJ. Bafoudiosbulbins A and B, two antisalmonellal clerodane diterpenoids from Dioscorea bulbifera var. sativa. Phytochemistry. 2006;67:1957–1963. doi: 10.1016/j.phytochem.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Teponno RB, Tapondjou AL, Jung HJ, Nam JH, Tane P, Park HJ. Three new clerodane diterpenoids from the bubils of Dioscorea bulbifera var. sativa. Helv Chim Acta. 2007;90:1599–1605. doi: 10.1002/hlca.200790168. [DOI] [Google Scholar]
- Teponno RB, Tapondjou AL, Abou-Mansour E, Stoeckli-Evans H, Tane P, Barboni L. Bafoudiosbulbins F and G, further clerodane diterpenoids from Dioscorea bulbifera L. var sativa and revised structure of Bafoudiosbulbin B. Phytochemistry. 2008;69:2374–2379. doi: 10.1016/j.phytochem.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Kuete V, Ngameni B, Tangmouo JG, Bolla J-M, Alibert-Franco S, Ngadjui BT, Pagès J-M. Efflux pumps are involved in the Gram negative bacterial defense against isobavachalcone and diospyrone, two natural products. Antimicrob Agents Chemother. 2010;54:1749–1752. doi: 10.1128/AAC.01533-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- Mativandlela SPN, Lall N, Meyer JJM. Antibacterial, antifungal and antitubercular activity of (the roots of) Pelargonium reniforme (CURT) and Pelargonium sidoides (DC) (Geraniaceae) root. S Afr J Bot. 2006;72:232–237. doi: 10.1016/j.sajb.2005.08.002. [DOI] [Google Scholar]
- Kuete V, Kamga J, Sandjo LP, Ngameni B, Poumale HM, Ambassa P, Ngadjui BT. Antimicrobial activities of the methanol extract, fractions and compounds from Ficus polita Vahl. (Moraceae) BMC Complement Altern Med. 2011;11:6. doi: 10.1186/1472-6882-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuete V, Ngameni B, Fotso Simo CC, Kengap Tankeu R, Tchaleu Ngadjui B, Meyer JJM, Lall N, Kuiate JR. Antimicrobial activity of the crude extracts and compounds from Ficus chlamydocarpa and Ficus cordata (Moraceae) J Ethnopharmacol. 2008;120:17–24. doi: 10.1016/j.jep.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Tereschuk ML, Riera MVQ, Castro GR, Abdala LR. Antimicrobial activity of flavonoid from leaves of Tagetes minuta. J Ethnopharmacol. 1997;56:227–232. doi: 10.1016/S0378-8741(97)00038-X. [DOI] [PubMed] [Google Scholar]
- Zgoda JR, Porter JR. A convenient microdilution method screening natural products against bacteria and fungi. Pharmaceut Biol. 2001;39:221–225. doi: 10.1076/phbi.39.3.221.5934. [DOI] [Google Scholar]
- Avila JG, de Liverant JG, Martínez A, Martínez G, Muñoz JL, Arciniegas A, de Vivar AR. Mode of action of Buddleja cordata verbascoside against Staphylococcus aureus. J Ethnopharmacol. 1999;66:75–78. doi: 10.1016/S0378-8741(98)00203-7. [DOI] [PubMed] [Google Scholar]
- Jimenez-Arellanes A, Meckes M, Ramirez R, Torres J, Luna-Herrera J. Activity against multidrug-resistant Mycobacterium tuberculosis in Mexican plants used to treat respiratory diseases. Phytother Res. 2003;17:903–908. doi: 10.1002/ptr.1377. [DOI] [PubMed] [Google Scholar]
- Teponno RB, Tapondjou AL, Djoukeng JD, Abou-Mansour E, Tabacchi R, Tane P, Lontsi D, Park HJ. Isolation and NMR assignment of a Pennogenin glycoside from Dioscorea bulbifera var sativa. Nat Prod Sci. 2006;12:62–66. [Google Scholar]
- Simões M, Bennett RN, Rosa EA. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat Prod Rep. 2009;26:746–757. doi: 10.1039/b821648g. [DOI] [PubMed] [Google Scholar]
- Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med. 2010;76:1479–1491. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- Kuete V, Efferth T. Cameroonian medicinal plants: pharmacology and derived natural products. Front Pharmacol. 2010;1:123. doi: 10.3389/fphar.2010.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims CA, Playfair JHL, Roitt IM, Wakelin D, Williams R, In: Med Microbiol Rev. 35. Mims CA, editor. 1993. Antimicrobials and chemotherapy; pp. 1–34. [Google Scholar]
- Nicolle LE. Infection control programmes to contain antimicrobial resistance. WHO/CDS/CSR/DRS/2001.7. http://whqlibdoc.who.int/hq/2001/WHOCDSCSRDRS2001.7.pdf. Accessed January 2009.
- Chevalier J, Pagès J-M, Eyraud A, Malléa M. Membrane permeability modifications are involved in antibiotic resistance in Klebsiella pneumoniae. Biochem Biophys Res Commun. 2000;274:496–499. doi: 10.1006/bbrc.2000.3159. [DOI] [PubMed] [Google Scholar]
- Savafi L, Duran N, Savafi N, Onlen Y, Ocak S. The prevalence and resistance patterns of Pseudomonas aeruginosa in intensive care units in a university hospital. Turk J Med Sci. 2005;35:317–322. [Google Scholar]
- Zager EM, McNerney R. Multidrug-resistant tuberculosis. BMC Infect Dis. 2008;8:10. doi: 10.1186/1471-2334-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub- Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet. 2006;367:926–927. doi: 10.1016/S0140-6736(06)68383-9. [DOI] [PubMed] [Google Scholar]
- Newton SM, Lau C, Gurcha SS, Besra GS, Wright CW. The evaluation of forty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria Canadensis. J Ethnopharmacol. 2002;79:57–67. doi: 10.1016/S0378-8741(01)00350-6. [DOI] [PubMed] [Google Scholar]
- Shriram V, Jahagirdar S, Latha C, Kumar V, Puranik V, Rojatkar S, Dhakephalkar PK, Shitole MG. A potential plasmid-curing agent, 8-epidiosbulbin E acetate, from Dioscorea bulbifera L. against multidrug-resistant bacteria. Int J Antimicrob Agents. 2008;32:405–410. doi: 10.1016/j.ijantimicag.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Gram-negative bacterial strains and features. The studied bacteria included reference ATCC strain of E. coli ATCC8739, E. aerogenes ATCC13048, K. pneumoniae ATCC12296 and P. aeruginosa PA01 as well as MDR strains E. coli AG100A and AG102, E. aerogenes EA-CM64 and EA289, K. pneumoniae Kp55 and Kp63, and P. aeruginosa PA124.