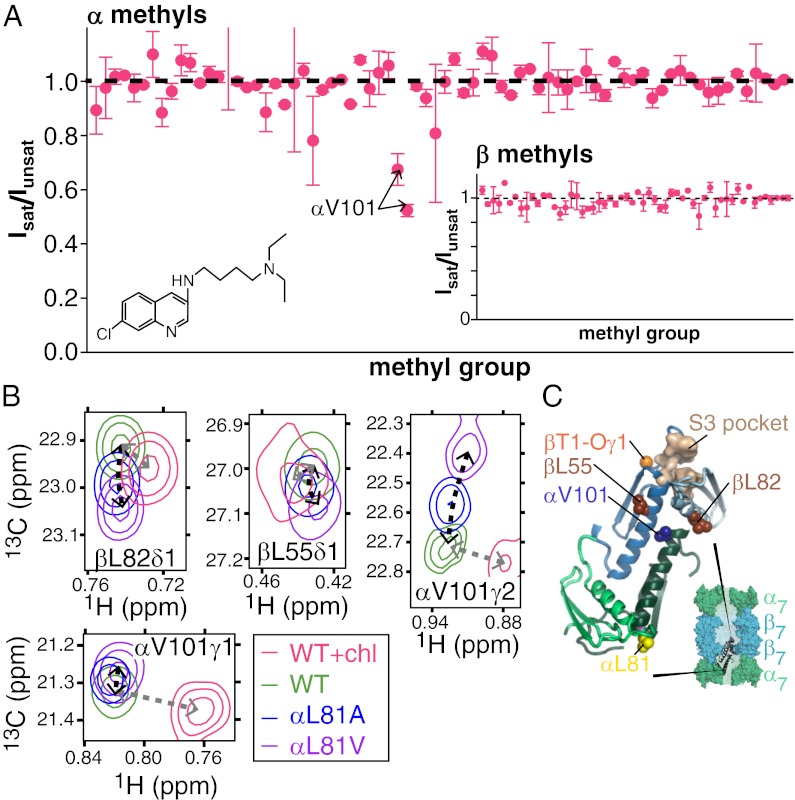
Fig. 7.
Location of the chloroquine binding site. (A) Saturation transfer experiments indicate that chloroquine (structure in Inset) binds near αV101. Samples consisted of chloroquine mixed with α, ILV-β or ILV-α, β CPs with equimolar concentrations of chloroquine and CP α/β-subunits. Peak intensities of CP methyl groups were compared in pairs of spectra acquired, with the center of the saturation bandwidth (±1 ppm) applied either at 7.9 ppm (Isat), which selectively saturates chloroquine aromatic protons, or at −6.5 ppm (Iunsat), a site approximately the same distance from the methyl groups as the aromatics. To minimize artifacts resulting from a small amount of saturation in the control (unsat) experiment or magnetization transfer that is unrelated to binding (49, 50), Isat/Iunsat has been renormalized by the intensity ratio obtained by measurement in an analogously prepared sample that lacked chloroquine. Renormalized values are plotted along the y axes. (B) CP methyl group chemical shifts indicate that the chloroquine binding site is located near αV101. Notably, several peaks shift in a manner distinct from the perturbations that occur in methyl groups along the allosteric pathway (Fig. 3), reflecting chloroquine binding. (C) Locations of residues whose methyl chemical shift changes reflect binding are shown, where the CP is depicted in a manner analogous to that in Fig. 4A.