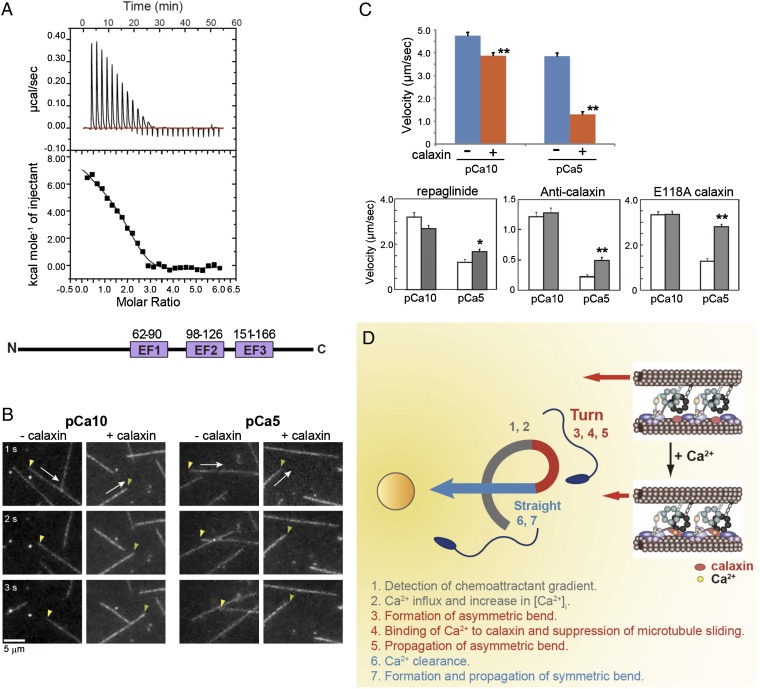
Fig. 4.
Ca2+ binding to calaxin suppresses dynein-driven microtubule translocation. (A) Isothermal titration calorimetry showing three binding sites for Ca2+ in calaxin. (Bottom) Location of three EF-hand Ca2+-binding motifs in calaxin. (B) Sequential dark-field images of microtubule translocation at pCa10 or pCa5 in the presence or absence of calaxin. Arrows indicate the direction of translocation. Arrowheads represent the minus (yellow) or plus (green) ends of microtubules. (C) Velocity of microtubule translocation. (Upper) Calaxin drastically suppresses translocation at pCa5. n = 73–129. (Lower) Repaglinide (n = 40–71), anti-calaxin antibody (n = 107–128), and a mutation in EF-hand 2 of calaxin (n = 89–95) cancel the calaxin-mediated suppression of microtubule translocation. Open bar, control; closed bar, presence of repaglinide, anti-calaxin antibody, or a calaxin mutant. *P < 0.01, **P < 0.001. (D) Proposed model of calaxin function in sperm chemotaxis. A chemoattractant induces Ca2+ influx and increased [Ca2+]i triggers asymmetry of the flagellar waveform. Ca2+ binding results in a conformational change in calaxin, its association with the dynein motor domain, suppression of microtubule sliding, and propagation of an asymmetric wave necessary for turn movement.