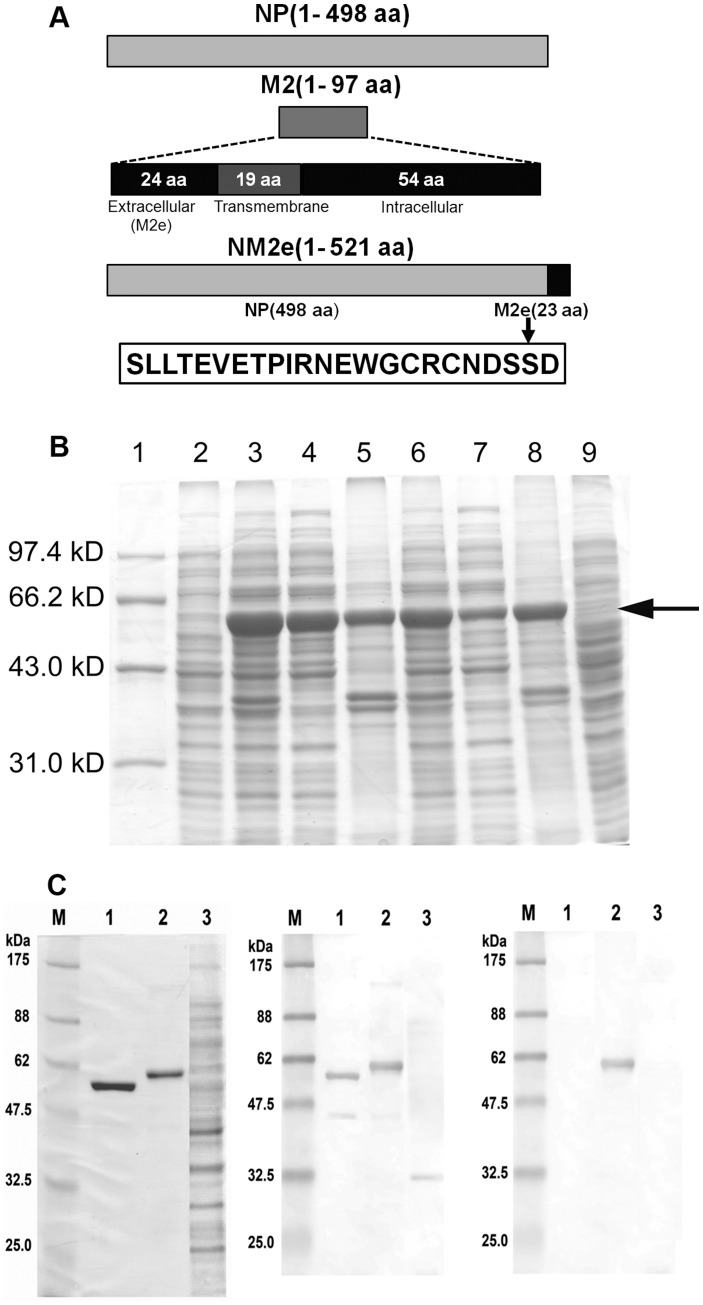
Figure 1. Construction, expression, and characterization of NM2e fusion protein.
(A) Schematic of influenza A virus NP and M2, and diagram for the construction of recombinant NM2e fusion protein. The entire sequence of NM2e is shown. The cDNA sequences encoding residues 1–498 of NP and residues 2–24 of M2 (extracellular domain of M2, M2e) from influenza A virus A/Beijing/30/95 (H3N2) were fused directly, with no linker, and this was cloned as pET30a-NM2e for expression in Escherichia coli. (B) Protein profile of cell lysates from induction experiments in E. coli BL21 (DE3) transformed with pET30a-NM2e at 25 and 37°C. Lane 1, Mid-range protein molecular weight marker; lane 2, whole-cell lysate of transformed E. coli before induction; lanes 3–5, whole-cell lysate, soluble supernatant, and insoluble fraction after 4-h induction with 0.1 mM IPTG at 25°C; lanes 6–8, whole-cell lysate, soluble supernatant, and insoluble fraction after 4-h induction with 0.1 mM IPTG at 37°C; lane 9, whole-cell lysate after 4 h of cultivation without IPTG at 37°C. The arrow indicates the 58-kDa band corresponding to NM2e. (C) SDS-PAGE (left) showing the purified NM2e fusion protein, NP of influenza A virus A/Beijing/30/95(H3N2), and lysates of E. coli transformed with pET30a(+). NM2e fusion protein was detected on Western blots probed with NP-immunized mouse serum (middle) and mouse anti-M2e monoclonal antibody (right). Lanes 1–3, purified influenza A virus NP, recombinant NM2e fusion protein expressed in E. coli, cell lysates of E. coli transformed with pET30a(+). M, protein molecular weight marker.