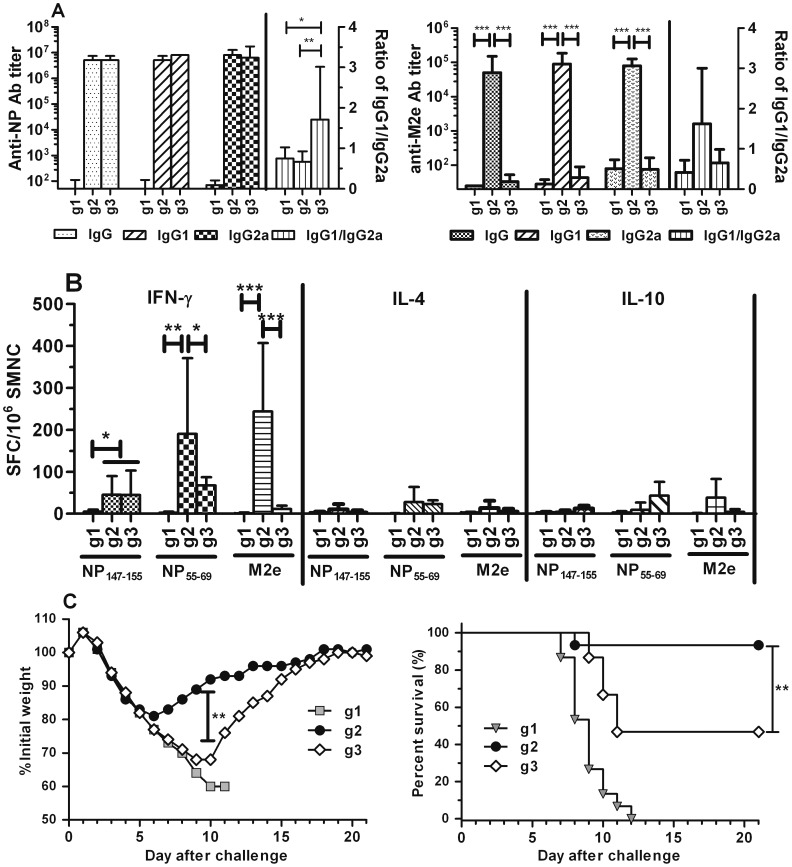
Figure 7. Comparison of the immunogenicity and protection efficacy induced by NM2e and NP.
Mice were immunized intramuscularly with 10 µg of NM2e (g2)or NP protein (g3) formulated with Al(OH)3 according to the time schedule in Fig. 2. Mice immunized with adjuvant alone were used as negative controls (g1). Serum, SMNC was prepared from each mouse and analyzed at the indicated times as described in the Materials and Methods. A), Ab and subtypes against NP (left) and M2e (right) on day 38. Columns show geometric mean antibody titers, and bars indicate the 95% confidence interval in each group (n = 6 mice per experimental group). B), SMNCs secreting IFN-γ, IL-4, or IL-10 upon stimulation were detected by ELISPOT assay. Six mice in each treatment group were sacrificed on day 38. The numbers of SMNCs producing IFN-γ (left), IL-4 (middle), or IL-10 (right) after stimulation for 40 h with NP147–155, NP55–69, or M2e peptides are presented as spot-forming cells (SFCs)/106 SMNCs. Columns show the average SFCs/106 SMNCs, and bars indicate the standard deviation of each group. *, p≤0.05; **, p≤0.01; ***, p≤0.001 by one-way ANOVA. C), Protective efficacy of immunization with NM2e or NP formulated with Al(OH)3 in mice. Three mice group were challenged with 20 LD50 of influenza virus PR8 (n = 15). Mice were monitored daily to detect morbidity (left) and mortality (right). *, p≤0.05; **, p≤0.01; ***, p≤0.001.