Table 1.
N-terminal coupling of IP scaffold 2a.
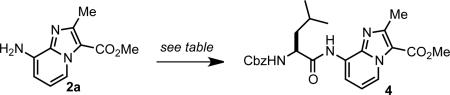
| ||
|---|---|---|
| entry | conditionsa | yieldb |
| 1 | 1.2 equiv Z-Leu-OH, 1.2 equiv HBTU, HOBt, NEt3, MeCN | trace |
| 2 | 1.2 equiv Z-Leu-OH, 1.2 equiv PyBOP, NEt3, DMF | trace |
| 3 | 1.2 equiv Z-Leu-OH, 1.2 equiv EDC, HOBt, NEt3, DCM | trace |
| 4 | 1.2 equiv Z-Leu-OH, 1.2 equiv EDC, 0.2 eq. DMAP, DCM | 33c |
| 5 | 2.0 equiv Z-Leu-OH, 2.0 equiv EDC, 0.2 eq. DMAP, DCM | 58c |
| 6 | 1.2 equiv Z-Leu-OH, 1.2 equiv EDC, DCM | 59 |
| 7 | 2.0 equiv Z-Leu-OH, 2.0 equiv EDC, DCM | 91 |
| 8 | 2.0 equiv Z-Leu-OH, 2.0 equiv PyBOP, DCM | 33 |
| 9 | 2.0 equiv Z-Leu-OH, 2.0 equiv COMU, DCM | 44 |
| 10 | 2.0 equiv Z-Leu-OH, 2.0 equiv DEPBT, DCM | 26 |
| 11 | 2.0 equiv Z-Leu-OH, 2.0 equiv DCC, DCM | 77 |
All reactions carried out for 24 h at rt.
Isolated yields.
HPLC analysis of a phenylalanyl derivitive showed significant epimerization of the Leu chiral center.
