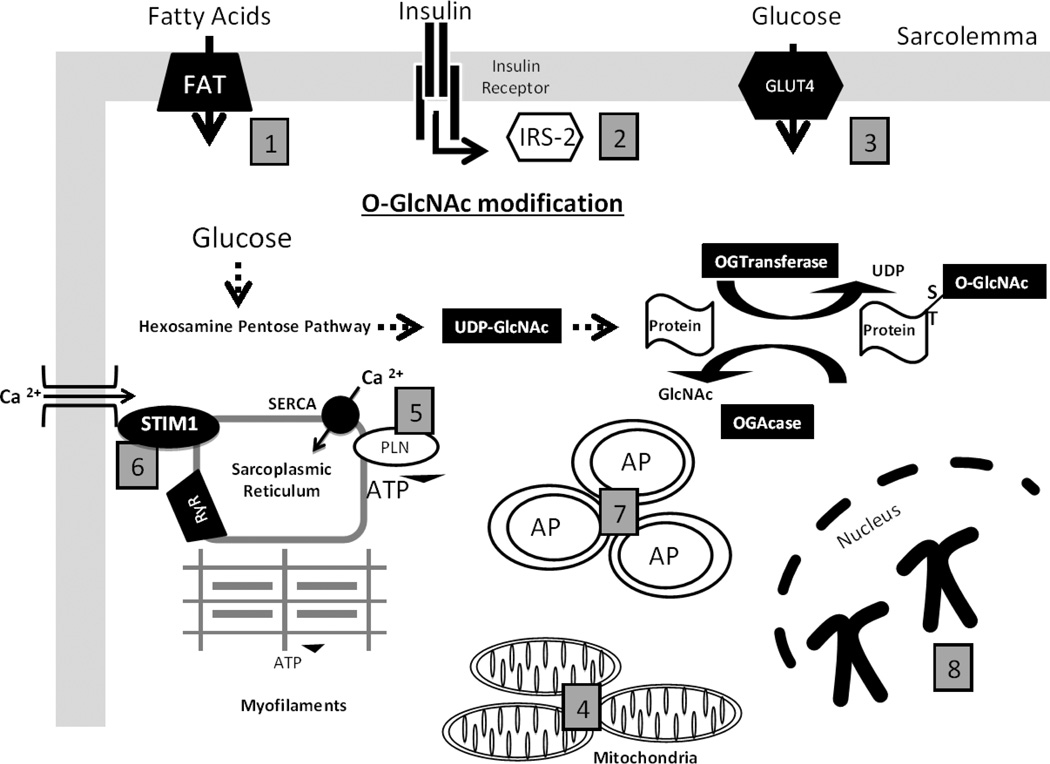
Figure 1. The roles of O-GlcNAc-mediated responses in the diabetic cardiomyocyte.
(1) Fatty Acid transporters (FAT36) is O-GlcNAc modified in the cardiomyocyte to shift substrate utilization in the diabetic heart. (2) Insulin Receptor Substrate (IRS) is O-GlcNAc modified to impair downstream insulin signaling and impair glucose transporters translocation to the membrane of the cardiomyocyte. (3) Glucose Transporter (GLUT) -4 is O-GlcNAc modified to impair glucose transport into the cardiomyocyte. (4) O-GlcNAcylation of mitochondrial proteins have been reported to inhibit mitochondrial respiratory capacity and increase mitochondrial fission, thereby impairing energy production. (5) Phospholamban (PLN) is O-GlcNAc modified to decrease Ca2+ removal by sarcroplasmic reticulum Ca2+ -ATPase (SERCA). (6) Stromal interacting molecule 1 (STIM1), a sarcroplasmic reticulum membrane protein, which regulates O-GlcNAc store operated calcium entry is O-GlcNAcylated and this may contribute to impaired cardiomyocyte Ca2+ signaling. (7) Autophagosomes (AP) are shown to represent potential O-GlcNAc regulation of autophagy, as Beclin-1 an early activator of autophagy is O-GlcNAcylated. (8) Chromosomes are representative of O-GlcNAc mediated regulation of chromatin, which are shown to modulate chromatin remodeling, gene transcription, and protein synthesis.