Table 2.
Iodolactonization of 6-Substituted-5(Z)-hexenoic Acidsa
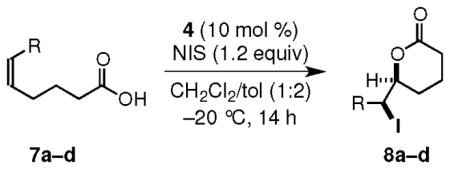
| ||||
|---|---|---|---|---|
| entry | R | product | yieldb (%) | erc,d |
| ae | Ph | 8a | 89 | 99:1 |
| bf,g | p-NC-C6H4 | 8b | 88 | 99:1 |
| cg | 2-Np | 8c | 93 | 98.5:1.5 |
| d | t-Bu | 8d | 98 | 98:2 |
Reactions run on 0.1 mmol scale.
Isolated yield after column chromatography.
er determined by chiral HPLC.
Absolute stereochemistry of 8a–d are based on analogy with 6c and 6e.
Z/E ratio of 7a, 14:1.
Z/E ratio of 7b, 20:1.
Results obtained after 38 h at −20 °C.
