Table 3.
Regiochemistry in Iodolactonization of 5-Substituted-4(E)-pentenoic Acidsa
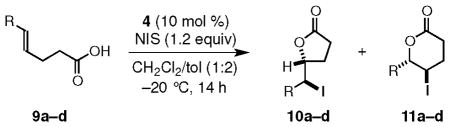
| ||||
|---|---|---|---|---|
| entry | R | product | yieldb (%) | erc,d |
| a | i-Pr | 10a | 78 | 67:33 |
| b | Ph | 10b+11b | 89e | 52:48(95:5)f |
| c | p-MeO-C6H4 | 11c | 89 | 86.5:13.5 |
| dg | p-NC-C6H4 | 10d | 94 | 58:42 |
Reactions run on 0.1 mmol scale.
Isolated yield after column chromatography.
er determined by chiral HPLC.
Absolute stereochemistry of iodolactones was assigned based on analogy with corresponding bromolactone analogs.3i
Combined yield; ratio of 1.3:1.0 based upon NMR analysis.
er shown in parentheses is for 11b,
Reaction performed for 14 h at −20 °C and 48 h at −10 °C in CH2Cl2/tol (1:1).
