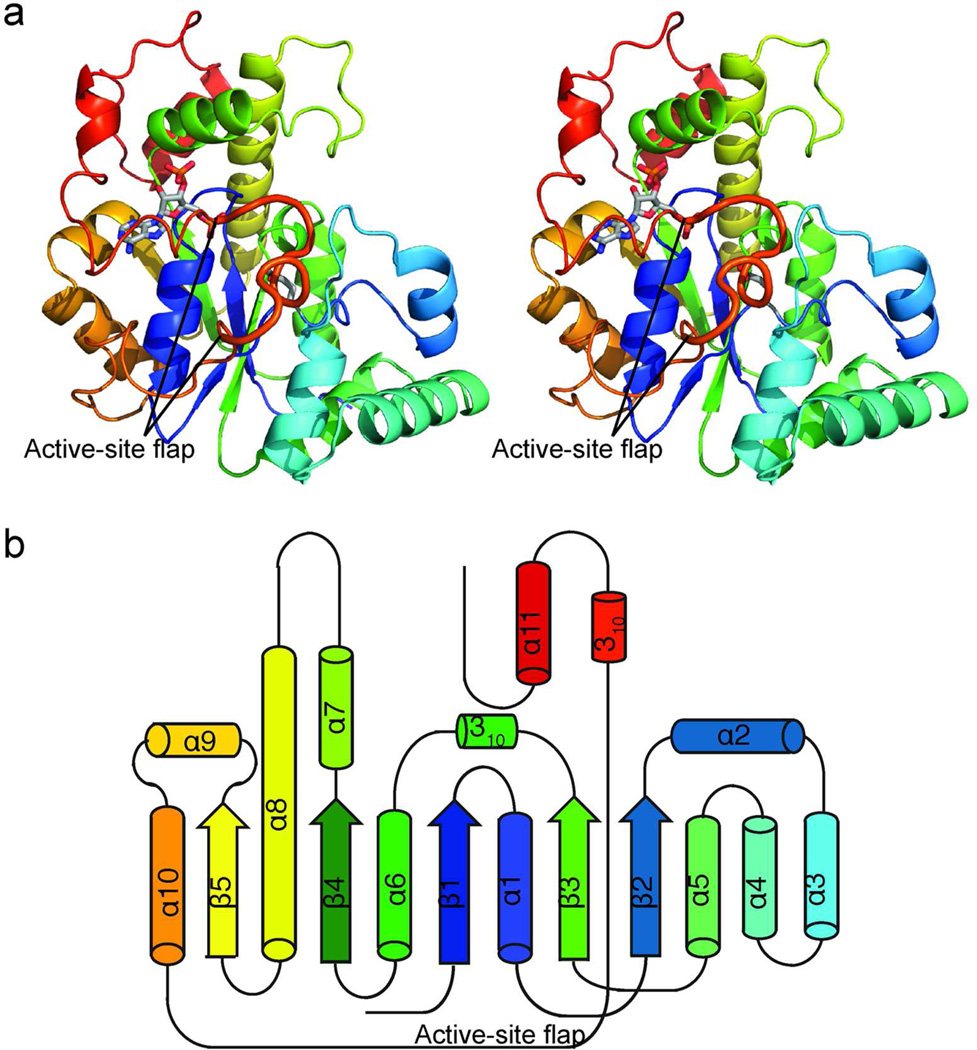
Figure 2.
Structure of activating STs. a) CurM ST polypeptide. The stereo ribbon diagram is colored as a rainbow from blue at the N-terminus to red at the C-terminus with PAP and Glu60, the proposed catalytic base, in stick (gray C). The active site flap (thick) is labeled. b) Topology diagram. CurM ST and OLS ST have an α/β core fold, like other STs, with inserted α-helices (α2–α4) and an extended loop wrapping around the core from α10–α11.