Abstract
Purpose
We aimed to characterize the association between levels of serum and follicular fluid (FF) adipocytokines, reflected by the leptin to adiponectin ratio (L:A ratio), and oocyte quality and in vitro embryo development in women undergoing assisted reproduction. We also aimed to assess whether follicular hormonal pathways mediate this interaction.
Methods
We prospectively collected FF from up to four individual preovulatory follicles (n = 76) and fasting sera from women (n = 31) without endocrinopathies undergoing in vitro fertilization (IVF) at a university-based center for assisted reproduction. Leptin, total adiponectin, insulin, insulin-like growth factor 1 (IGF-1), and ovarian steriods were measured using enzyme immunoassay. Oocyte maturity, fertilization, and embryo development were assessed.
Results
FF leptin was similar to serum levels while FF adiponectin was lower. FF leptin (27.10 ± 4.05 ng/mL) and the L:A ratio (11.48E−3 ± 2.57E−3) were related to FF insulin (R2 = 0.370 and 0.419, p < 0.001) but not to ovarian steroids or IGF-1, whereas FF adiponectin ( 4.22 ± 0.52 ug/mL) correlated only with leptin (R2 = −0.138, p = 0.001). Oocytes from a high FF L:A ratio environment were 81 % (RR 1.81 [95%CI 0.97–3.37]) more likely to undergo successful cleavage and 117 % (RR 2.17 [95 % CI 1.06–4.44]) more likely to obtain viable cleavage morphology compared to a low FF L:A ratio environment, even when adjusted for FF insulin, an independent predictor of cleavage.
Conclusions
Certain adipocytokines, particularly the L:A ratio in the FF of the preovulatory follicle, are related to successful in vitro embryo development. This action may be independent of FF insulin.
Keywords: Adipocytokines, Leptin, Adiponectin, Follicular fluid, IVF, Oocyte quality, Embryo development, Steroidogenesis, Insulin
Introduction
Over the past decade, it has become increasingly apparent that the regulation of body weight is tightly linked to female reproductive function. How body weight affects reproduction is very complex and not well understood. However, this regulation appears to be directly controlled by fat mass, a previously underappreciated endocrine organ.
The effects of adiposity on reproduction are partly mediated by a host of protein hormones secreted by adipose tissue, collectively called adipocytokines, of which leptin and adiponectin are two representative members. Leptin is positively correlated with body weight and insulin resistance (IR). In contrast, adiponectin has anti-inflammatory and insulin sensitizing properties, and is negatively associated with weight [25, 26]. Together, they regulate long term satiety and energy expenditure [11]. Moreover, the ratio of leptin to adiponectin (L:A ratio) is a stronger indicator of abdominal adiposity and IR than either component alone [14, 25].
Studies have shown that both leptin and adiponectin can regulate the hypothalamic pituitary gonadal axis independently, as well as synergistically through their interactions with each other and with other metabolically active hormones [11, 18]. Specifically at the level of the human ovary, adipocytokines are implicated in the regulation of steroidogenesis by modulating the action of insulin and insulin like growth factor 1 (IGF-1) [5, 12]. A reasonable next question is whether ovarian adipocytokines influence human oocyte and embryo quality. Follicular fluid leptin has been associated with improved oocyte fertilization [9] and leptin supplementation in culture, among other growth factors, may promote the development of parthenogenic embryos [17]. Although adiponectin receptors have been identified in the human ovary, it is unclear whether adiponectin itself is expressed [20]. In vitro studies have shown that adiponectin and its receptors can regulate the expression of genes that are important in steroidogenesis and ovulation [5, 15, 20]. Because of low adiponectin expression by human granulosa cells, it remains a question the role of adiponectin on human oocyte quality and early embryo development. Furthermore, very few studies have examined the composite and synergistic actions of leptin and adiponectin together as in the case of the L:A ratio on the female reproductive system.
This study was designed to investigate the association between ovarian adipocytokines, with special attention to the L:A ratio as a composite marker, and human oocyte quality and in vitro embryo development in the setting of ovarian stimulation. Follicular fluid, a medium rich with sex steroids, gonadotropins, micronutrients, and growth factors, nourishes the maturing oocyte [19], whose quality then determines early embryo development [4]. Based on existing literature and our conceptual understanding of adipocytokine function, our primary hypothesis was that preovulatory intrafollicular L:A levels are positively associated with the oocyte’s ability to develop into viable early stage in vitro fertilized embryos. We further hypothesized that the follicular fluid hormonal milieu, including sex steroids, insulin, and IGF-1, may interact with adipocytokines and mediate this potential action.
To address these hypotheses, we prospectively examined the levels of adipocytokines in individual preovulatory dominant follicles from women without endocrinopathies undergoing ovarian stimulation, and compare in vitro embryo development among oocytes associated with increasing levels of FF leptin, adiponectin, and the L:A ratio. Second, we assessed whether the association between adipocytokines and embryo development was dependent on follicular concentrations of 17β estradiol (E2), testosterone (T), progesterone (P), insulin, and IGF-1.
Materials and methods
Ovarian stimulation, oocyte aspiration, and embryo transfer
The Institutional Review Board at Columbia University Medical Center approved this study. After informed consent, women (n = 31) presenting for autologous in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) between February and December 2010 were recruited via systemic random sampling. Patients with endocrinopathies such as polycystic ovary syndrome (PCOS) or hypothalamic amenorrhea were excluded. Based on clinical indications, ovarian stimulation was achieved using purified or recombinant FSH and human menopausal gonadotropins (hMG) with pituitary down-regulation using GnRH agonist or antagonist. Once three or more follicles had reached 18–20 mm in diameter, patients self-administered 10,000 IU of human chorionic gonadotropin (hCG) (Novarel; Ferring Pharmaceuticals Inc., Parsippany, NJ) IM to trigger ovulation. Transvaginal oocyte aspiration was carried out 34 h later with a 17-gauge single lumen needle. Day 3 embryo transfer (ET) was performed unless the patient was selected as a candidate for a blastocyst transfer, which was based on individual patient characteristics and the number of available viable cleavage stage embryos (see below). Daily vaginal progesterone (200 mg) provided luteal support. Biochemical pregnancy was determined by a positive serum hCG 10–12 days after ET. Transvaginal ultrasound was performed at 6 weeks gestation to identify the presence of intrauterine gestational sacs, which defined clinical pregnancy.
Hormone levels of up to four dominant follicles from each patient and embryo development of the corresponding oocytes were analyzed for this study.
Biological sample collection
For each participant, 2–5 mL of clear FF was aspirated individually from up to four dominant follicles containing oocyte-corona-cumulus (OCC) complexes (n = 76 follicles). The needle was removed, flushed with culture media, and drained completely between each aspirate to minimize contamination. After centrifugation at 3,000 rpm for 10 min at 4 °C to separate cells, blood, and debris, the supernatant was stored at −20 °C until assay. Sera on cycle day 2 (baseline) prior to ovarian stimulation and after an overnight fast on the day of oocyte aspiration (midcycle) were obtained and stored similarly.
Hormone measurements
All hormones were measured in duplicate using enzyme immunoassay (EIA) (total adiponectin: Millipore Co., Billerica, MA; leptin: R&D Systems, Minneapolis, MN; E2, T, P, insulin, and IGF-1: Immulite Siemens Healthcare Diagnostics, Los Angeles, CA; anti-mullerian hormone (AMH): Beckman/Diagnostic Systems Lab Inc., Brea, CA). Intra-assay and inter-assay coefficients of variation (CV) for all hormones with the exception of E2 were <10 %. The inter-assay CV for E2 was 10.5 %. FF leptin tertiles were defined as low (<11.87 ng/mL), medium (≥11.87 ng/mL and <40.63 ng/mL), and high (≥40.63 ng/mL). FF adiponectin tertiles were defined as low (<2.16 μg/mL), medium (≥2.16 μg/mL and <4.63 μg/mL), and high (≥4.63 μg/mL). FF L:A tertiles were defined as low (<3.31E−3), medium (≥3.31E−3 and <19.79E−3), and high (≥19.79E−3).
Oocyte/embryo outcome assessment
The oocytes and embryos corresponding to the study follicles were cultured individually in separate dishes. Information on oocyte maturity, fertilization, and daily embryo morphology was recorded. A mature oocyte was defined as being in metaphase II of meiosis (MII). ICSI was performed on all MII oocytes. Normal fertilization was assessed by the presence of 2 pronuclei (2PN) 16–18 h after injection. Embryos were graded according to the Society for Assisted Reproductive Technology (SART) system. For this study, successful cleavage was defined as having ≥6 cells by day 3 after fertilization and successful blastulation defined as being an expanded or hatching blastocyst by day 6. Viable morphology at either stage was defined as having an overall grade of “good” or “fair”.
Statistical analysis
Statistics were carried out using IBM© SPSS© Statistics 19.0.0 (IBM Corporation, Somers, NY). Using α = 0.05 and 80 % power, we calculated a total sample size of 39 oocytes for 3 groups of FF adipocytokine levels to see a relative risk of 50 % for successful cleavage and 108 oocytes for a relative risk of 30 %. Linear relationships were evaluated using the Pearson bivariate correlation, partial correlation and multiple linear regression analysis for adjustment of covariates. Comparisons between groups were calculated using the Student t test (independent or paired sample) or 1-way ANOVA. Extended Mantel-Haenszel Chi Square and relative risk (RR) were used to calculate association between increasing levels of FF adipocytokines and embryo development. In cases involving non-normally distributed variables such as FF and serum leptin, FF and serum adiponectin, FF and serum L:A ratio, FF and serum insulin, and baseline AMH, natural log (Ln) transformation was performed prior to analysis. Additionally, calculations were repeated using the corresponding nonparametric methods such as the Spearman correlation, the Mann–Whitney U test, Wilcoxon signed-rank, and Kruskal Wallis 1-way ANOVA for confirmation. All tests were two-tailed.
Results
Subjects and outcomes
The participants were between 29 and 44 years of age (median 36 years) with an average weight of 65.42 ± 2.94 kg (mean ± SE) and body mass index (BMI) 24.04 ± 0.99 kg/m2. Mean baseline AMH was 2.03 ± 0.51 ng/mL and FSH was 6.05 ± 0.62 mIU/mL. In terms of cycle outcomes, the mean number of aspirated oocytes was 11.90 ± 1.25; 78.0 % ± 3.5 % were MII; 69.3 % ± 3.6 % of MII oocytes were normally fertilized with the presence of 2PN; and 4.55 ± 0.62 viable cleavage stage embryos were obtained per cycle. 41.9 % of cycles met criteria for blastocyst transfer resulting in 2.75 ± 0.62 viable blastocysts per cycle. The average number of embryos transferred per cycle was 2.57 ± 0.26. The biochemical pregnancy rate (per ET) was 47.8 % and the clinical pregnancy rate was 39.1 %.
Serum adipocytokines and correlates
Leptin and adiponectin
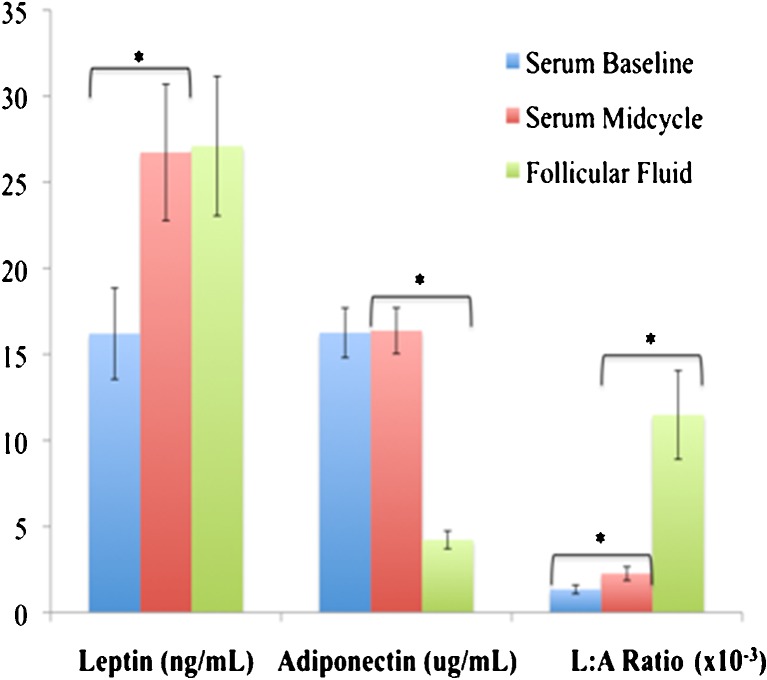
Serum leptin levels rose significantly (p < 0.001) from baseline (16.20 ± 2.65 ng/mL) to midcycle (26.73 ± 3.96 ng/mL) suggesting perhaps a stimulatory effect by exogenous gonadotropins either directly or indirectly, whereas serum adiponectin levels remained unchanged (16.37 ± 1.33 μg/mL vs. 16.25 ± 1.44 μg/mL, p = 0.514, Fig. 1). As expected, both weight and BMI correlated positively with serum leptin (r = 0.724, p < 0.001; r = 0.753, p < 0.001; respectively) and correlated negatively with serum adiponectin (r = −0.384, p = 0.036; r = −462, p = 0.010; respectively).
Fig. 1.
Mean [± SE] levels of adipocytokines in serum at baseline, midcycle, and in the preovulatory follicular fluid (FF). Serum midcycle leptin was higher than on baseline (p < 0.001), but it was not different from FF (p = 0.35, Student t test). FF adiponectin was different from that of midcycle serum (p < 0.001, Student t test). The L:A ratios were different between serum at baseline and midcycle, as well as between serum midcycle and FF (p < 0.001 for both comparisons, paired t test). Note: All hormones were natural log (LN) transformed to achieve normal distribution prior to comparison. * Denotes statistical significance at p < 0.05
The L:A ratio
Due to the differential change in serum leptin and adiponectin from baseline to midcycle, the serum L:A ratio increased more than 70 % (ratio 1.72, 95 % CI 1.46–1.80, the midcycle serum mean 2.26E-3 ± 0.40E-3 vs. the baseline serum mean 1.34E-3 ± 0.25E-3, p < 0.001, Fig. 1). Weight and BMI correlated positively with the serum L:A ratio (r = 0.695, p < 0.001; r = 0.743, p < 0.001; respectively).
Hormonal correlates
Multiple linear regression analyses of all the midcycle serum hormones measured (leptin, adiponectin, E2, insulin, and IGF-1) showed that insulin (as the independent variable) predicted midcycle serum leptin (dependent variable, regression coefficient B = 0.664, [95 % CI 0.260–1.168], β = 0.529, R2 = 0.280, p = 0.002) and the L:A ratio (dependent variable, B = 0.902, [95 % CI 0.358–1.445], β = 0.533, R2 = 0.284, p = 0.002). With midcycle serum adiponectin as the dependent variable, regression showed that only serum leptin (as the independent variable) was correlated (B = −0.252, [95 % CI −0.442 – −0.062], β = −0.449, R2 = 0.202, p = 0.011). Serum IGF-1 was not related to adipocytokines (data not shown).
Follicular fluid adipocytokines and correlations
FF leptin and adiponectin
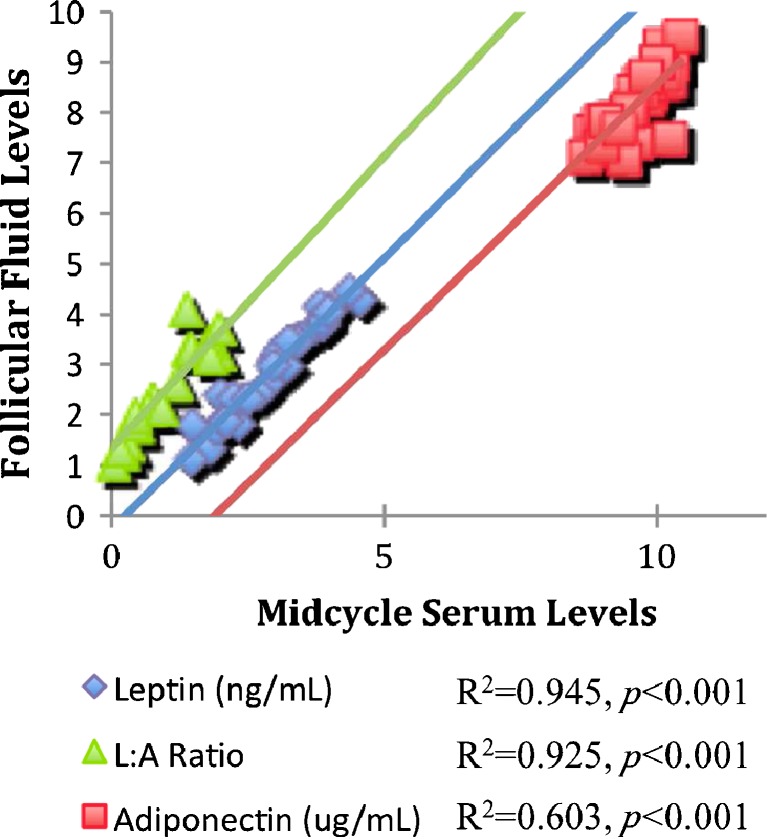
Mean FF leptin was 27.10 ± 4.05 ng/mL and equal to midcycle serum levels (p = 0.350, Fig. 1). There was a strong positive correlation between the two (Fig. 2), suggesting that FF leptin was likely to be a serum transudate. Mean FF adiponectin (4.22 ± 0.52 μg/mL) also showed a strong correlation with midcycle serum adiponectin (Fig. 2). However, FF adiponectin levels were significantly less than serum levels (p < 0.001, Fig. 1), suggesting that adiponectin was not produced locally in the ovaries and that it has limited transudation in the follicular compartment.
Fig. 2.
Linear regressions between follicular fluid (FF) and midcycle serum adipocytokines. Leptin: constant = −0.27 (95 % confidence interval [CI] −0.57–0.04), B = 1.08 (95 % CI 0.98–1.18), R2 = 0.945, p < 0.001. Adiponectin: constant = −1.98 (95 % CI −5.10–1.15), B = 1.06 (95 % CI 0.73–1.38), R2 = 0.603, p < 0.001. The L:A ratio: constant = 1.34 (95 % CI 1.19–1.48), B = 1.16 (95 % CI 1.03–1.28), R2 = 0.925, p < 0.001. Note: All hormones were natural log (LN) transformed to achieve normal distribution prior to statistical analysis
The FF L:A ratio
As expected, the FF L:A ratio (11.48E-3 ± 2.57E-3) correlated strongly with the midcycle serum ratio (Fig. 2). However, the L:A ratio was significantly higher in FF than in serum (p < 0.001, Fig. 1). This marked increase was the result of the differential change in the concentrations of leptin and adiponectin from serum to FF, and the selective increase in the midcycle serum leptin as the result, directly or indirectly, of gonadotropin stimulation.
FF hormonal correlates
Among all measured FF hormones (leptin, adiponectin, E2, T, P, insulin, and IGF-1), FF insulin levels correlated positively with FF leptin (B = 1.141 [95 % CI 0.828–1.454], β = 0.653, R2 = 0.419, p < 0.001) and the FF L:A ratio (B = 1.524 [95 % CI 1.062–1.986], β = 0.615, R2 = 0.370, p < 0.001). FF adiponectin correlated with FF leptin levels (B = −0.260 [95 % CI −0.413 – −0.106], β = −0.372, R2 = 0.138, p = 0.001). FF steroids and IGF-1 were not associated with FF adipocytokines.
Serum adipocytokines and cycle outcomes
Leptin and adiponectin and outcomes
Midcycle serum adiponectin correlated negatively with the number of total oocytes aspirated (r = −0.482, p = 0.006), the number of cleavage stage embryos (r = −0.504, p = 0.004), the number of viable cleavage embryos (r = −0.497, p = 0.004), and the cleavage rate (r = −0.524, p = 0.002). Midcycle serum adipoenctin also showed a trend towards negative correlation with the number of mature oocytes aspirated (r = −0.333, p = 0.067), although the correlation did not reach statistical significance. When adjusted for potential confounders (age, BMI, peak serum insulin, E2, and baseline serum AMH), the correlations between midcycle serum adiponectin and the total number of oocytes became stronger (r = −0.524, p = 0.026) while the correlations with cleavage development remained similar or weaker (cleavage embryo r = −0.514, p = 0.029; viable cleavage embryo r = −0.481, p = 0.044; cleavage rate r = −0.424, p = 0.080). Serum leptin was not related to any cycle outcomes.
The L:A ratio and outcomes
The serum L:A ratio, representing the combined effects of leptin and adiponectin, correlated positively with the cleavage rate (r = 0.380, p = 0.035). Interestingly, when adjusted for age, peak serum insulin, E2, and AMH, the associations with the total number of oocytes and the number of cleavage embryos became stronger and statistically significant (r = 0.537, p = 0.018; r = 0.472, p = 0.041; respectively), whereas the negative associations became statistically non-significant when adjusted for BMI. Furthermore, the serum L:A ratio was higher (p = 0.013) in cycles that resulted in ≥8 embryos reaching the cleavage stage (4.44E-3 ± 0.83E-3) compared to those <8 (1.84E-3 ± 0.41E-3); and there was a trend (p = 0.062) towards a higher serum L:A ratio in cycles that were candidates for blastocyst transfer (3.01E-3 ± 0.66E-3) compared to cycles that were not (1.72E-3 ± 0.48E-3). Serum adipocytokine levels did not correlate with the pregnancy rates.
Follicular fluid adipocytokines and oocyte/embryo outcomes
FF leptin and adiponectin
The quality and development of individual oocytes were tracked through the cleavage stage (76 oocytes from 31 cycles) and blastulation (37 oocytes from 13 blastocysts-only cycles). Extended Mantel-Haenszel Chi Square for linear trend was calculated to assess if increasing tertiles of FF adipocytokines affected embryo development outcomes. There was a trend towards improved cleavage and blastocyst morphology with increasing FF leptin tertiles (χ2 = 3.45, p = 0.063; χ2 = 4.96, p = 0.026, respectively). In contrast, increasing FF adiponectin was negatively associated with viable cleavage morphology (χ2 = 3.92, p = 0.048), successful blastulation (χ2 = 7.27, p = 0.007), and viable blastocyst morphology (χ2 = 4.03, p = 0.045).
The FF L:A ratio
Oocyte/embryo outcomes based on low, medium, and high FF L:A tertiles are listed in Table 1. The FF L:A ratio, representing the combined effects of FF leptin and adiponectin, was positively associated with normal cleavage and blastulation (Table 1). To further assess the relationship between the FF L:A ratio and embryo development at the cleavage stage, we calculated unadjusted relative risks of cleavage and viable morphology according to the FF L:A tertiles. The normally fertilized oocytes that came from medium to high FF L:A environments were 49 %–81 % more likely to cleave successfully and 63 %–117 % more likely to obtain viable morphology by day 3 (Table 2).
Table 1.
Outcomes of oocyte quality and embryo development across the FF L:A ratio tertiles
| Embryological outcomes | Low FF L:A | Med FF L:A | High FF L:A | All | P |
|---|---|---|---|---|---|
| Age (years) | 37.6 ± 5.1 | 35.6 ± 4.0 | 36.4 ± 3.2 | 36.6 ± 4.2 | 0.555 |
| BMI (kg/m2) | 20.4 (4.4) | 23.4 (2.5) | 27.3 (13.3) | 23.4 (4.3) | 0.036 |
| Baseline serum AMH (ng/mL) | 2.4 (2.6) | 1.2 (1.2) | 2.3 (1.9) | 1.6 (2.0) | 0.530 |
| Peak serum estradiol (pg/mL) | 2384 ± 1033 | 1717 ± 792 | 2259 ± 1530 | 2114 ± 1113 | 0.339 |
| Oocytes studied N | 24 | 25 | 25 | 74 | |
| Mature (MII) oocytes N (% total) | 22 (91.7) | 23 (92.0) | 23 (92.0) | 68 | 0.796 |
| Normal fertilization (2PN) N (% MII) | 13 (59.1) | 16 (69.6) | 18 (78.3) | 47 | 0.223 |
| Successful cleavage N (% 2PN) | 6 (46.2) | 11 (68.8) | 15 (83.3) | 32 | 0.050 |
| Viable cleavage morphology N (% 2PN) | 8 (38.5) | 10 (62.5) | 15 (83.3) | 30 | 0.019 |
| Successful blastulation N (% 2PN) | 0 | 2 (33.3) | 11 (78.6) | 13a | 0.028a |
| Viable blastocyst morphology N (% 2PN) | 0 | 2 (33.3) | 2 (14.3) | 4a | 0.574a |
AMH = anti-mullerian hormone; FF = follicular fluid; the L:A ratio = leptin to adiponectin ratio; Med = medium; MII = meiosis II; 2PN = 2 pronuclei. Values are reported as mean ± standard deviation for age and peak serum estradiol, as median (interquartile range) for body mass index and baseline serum AMH, and as number and percent for all other rows. P values were calculated using the One-Way Anova for age and peak serum estradiol, the nonparametric median test for body mass index and baseline serum AMH, the extended Mantel-Haenszel Chi Square test for linear trend for oocyte and embryo outcomes. The FF L:A tertiles are separated by 3.71E−3 and 19.79E−3
aFrom 13 blastocyst-only cycles (37 total oocytes and 23 normally fertilized oocytes)
Table 2.
Unadjusted and adjusted relative risks of embryo development with the FF L:A ratio tertiles
| Unadj RR | 95 % CI | Adj RR | 95 % CI | |
|---|---|---|---|---|
| Successful cleavage | ||||
| Low FF L:A ratio | 1 (ref) | – | 1 (ref) | – |
| Med FF L:A ratio | 1.49 | 0.76–2.92 | 1.03 | 0.56–1.90 |
| High FF L:A ratio | 1.81 | 0.97–3.37 | 1.53 | 1.01–2.32 |
| Viable cleavage morphology | ||||
| Low FF L:A ratio | 1 (ref) | – | 1 (ref) | – |
| Med FF L:A ratio | 1.63 | 0.74–3.56 | 1.13 | 0.42–3.01 |
| High FF L:A ratio | 2.17 | 1.06–4.44 | 2.22 | 1.08–4.55 |
FF = follicular fluid; L:A ratio = leptin to adiponectin ratio; RR = relative risk; CI = confidence interval. The FF L:A tertiles are separated by 3.31E−3 and 19.79E−3. Mantel-Haenszel adjusted RR were computed for strata of low and high FF insulin (<50th percentile vs. ≥50th percentile, 50th percentile for FF insulin was 4.24 uIU/mL)
As an independent hormone predictor of FF L:A ratio, FF insulin was found to be positively associated with embryo development. FF insulin levels were higher in successfully cleaved and viable embryos (5.27 ± 0.58 μIU/mL vs. 3.68 ± 0.49 μIU/mL, p = 0.040; 5.26 ± 0.50 μIU/mL vs. 3.86 ± 0.47 μIU/mL, p = 0.088; respectively). Additionally, the relative risk of cleavage with high FF insulin (≥4.245 μIU/mL, 50th percentile) compared to low FF insulin (<4.245 μIU/mL, <50th percentile) was 1.53 (95 % CI 1.02–2.37), and the relative risk of viable morphology was 1.60 (95 % CI 1.01–2.54).
In order to assess whether the association between the FF L:A levels and embryo development at the cleavage stage was due to the influence of FF insulin, we calculated the adjusted relative risks, using the Mantel-Haenszel method to adjust for FF insulin, of embryos in cleavage stage and with viable morphology in an environment of medium to high FF L:A ratios as compared to a low FF L:A ratio, We showed that the relative risks remained essentially unchanged and statistically significant (Table 2). This suggested that the FF L:A ratio and FF insulin independently associate with embryo development at the cleavage stage. Relative risks of blastulation according to the FF L:A tertiles were not calculated due to small numbers.
Discussion
This study is the first to characterize the L:A ratio in serum and FF of reproductive aged women in the setting of ovarian stimulation. We showed that, secondary to a leptin increase, the serum L:A ratio increased with exogenous gonadotropins. Given that serum and follicular levels of leptin were tightly correlated and essentially equivalent, we concluded that intrafollicular levels were derived mostly from serum transudation, and very little if at all from ovarian production, despite the known expression of leptin and its receptor in human pre-ovulatory follicles [7]. In the linear regression model, FF insulin predicted FF leptin and the L:A ratio independently, while it indirectly correlated with FF adiponectin via leptin. Both FF adipocytokines, leptin positively and adiponectin negatively, associated with successful cleavage as well as viable morphology. Consequently, the overall relationship between the FF L:A ratio and embryo development was positive. After adjusting for the influence of FF insulin as a covariate, it appeared that FF adipocytokines are linked to early in vitro embryo development via insulin independent mechanisms.
Methodologically, two details must be noted. First, we measured fasting levels of insulin to minimize prandial fluctuations and fasting levels have been shown to correlate better with leptin [8]. Second, we measured total adiponectin instead of its various polymers. Human adiponectin is a 244aa/30 kDa protein that circulates as trimers (low molecular weight, ~10 % of total), hexamers (medium molecular weight, ~40 %), and 12–18-mers (high molecular weight, ~50 %) in reproductively aged women [16, 21]. Since it is unclear which form is biologically active on the level of the ovary [21], we decided that total adiponectin would give us the most comprehensive results. Moreover, total and higher molecular weight forms trend in the same direction in their relationships to female sex steroids and insulin sensitivity [16].
Our descriptive data on the behaviors of serum and follicular adipocytokines in the setting of gonadotropin stimulation were consistent with what has been reported in the literature. First, both leptin and adiponectin correlated in opposite directions with body weight and BMI as expected [1, 8]. Second, we confirmed that exogenous gonadotropins have a stimulatory effect, either directly or indirectly, on serum leptin levels [23] but had a minimal effect on adiponectin [3]. Third, the levels of adiponectin were very low in the preovulatory follicular fluid consistent with absent or undetectable expression of this protein in human granulosa cells [20]. Lastly, it is well established that insulin can stimulate leptin production both in vivo and in vitro [13], whereas adiponectin may potentiate insulin sensitivity at the tissue level but not necessarily affect its expression [2, 22]. This is consistent with our observation that FF leptin and the L:A ratio positively correlated with FF insulin, while adiponectin and insulin were indirectly associated.
Confirming our primary hypothesis, the FF L:A ratio was associated with improved potential for early in vitro embryo development. These results may be counterintuitive at first glance because hyperleptinemia and hypoadiponectinemia are considered “high risk” markers for metabolic pathology [14]. However, we would argue that pathological states of metabolism such as obesity and IR are inherently different from those in endocrinologically normal women. There is evidence that at least the insulin leptin relationship is different in normal and obese subjects [8]. Furthermore, leptin at physiological levels is a signal of energy abundance whereas adiponectin can be thought of as functionally the opposite [11]. Conceptually, it stands to reason that the energy abundant state, as reflected by a high L:A ratio, would provide a positive environment for embryo development.
Lastly, the influence of ovarian adipocytokines on embryo development was independent of insulin suggesting that we need to consider the possibility of non-classical mechanisms. We hypothesize that intrafollicular adipocytokines directly modulate gene expression of the cumulus-oocyte-complex, and via alternative downstream signaling pathways other than that of insulin. There is evidence that both leptin and adiponectin receptors are expressed in the cumulus cells, and adiponectin receptors exhibit differential target gene expression profile in cumulus (CC) compared to granulosa cells (GC) [20, 24]. One example of an interesting target gene is Bmp2, which has been implicated in follicular apoptosis and fetal germ cell differentiation in the ovary [6, 10]. Adiponectin was found to upregulate the expression of Bmp2 in CCs [20], although whether the adiponectin Bmp2 interaction has a functional significance in vivo remains to be elucidated. Similarly, the presence of leptin receptors in CCs suggests a direct role of leptin on oocyte regulation. It has been described that leptin signaling in CCs may involve growth differentiation factor 9 (GDF9) and pentraxin 3 (PTX3), factors important in cumulus expansion which is crucial for oocyte developmental competence and fertilization [24]. Lastly, it remains to be studied whether leptin and adiponectin and their receptors are expressed on the oocyte itself to directly modulate growth and maturation.
In conclusion, our data demonstrated that follicular composition of adipocytokines differ greatly from that of serum secondary to differential diffusion ability of leptin and adiponectin across the blood follicle barrier, and there is little or no de novo hormone production in the ovary. Nevertheless, the intrafollicular L:A ratio is positively associated with early embryo development, presumably by modulating oocyte quality. Although the FF L:A ratio affects the action of follicular insulin, its relationship to embryo development is likely mediated by a separate and unknown mechanism. This evidence lends support to our overall hypothesis that metabolism and reproduction are inextricably linked. Further investigation should focus on the effects of endogenous adipocytokines on blastocyst development as well as in patients with metabolic dysregulation such as obesity and PCOS.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Capsule
The intrafollicular leptin to adiponectin ratio positively correlates with early in vitro embryo development independent of insulin.
References
- 1.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 2.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7(8):947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 3.Bersinger NA, Birkhäuser MH, Wunder DM. Adiponectin as a marker of success in intracytoplasmic sperm injection/embryo transfer cycles. Gynecol Endocrinol. 2006;22(9):479–483. doi: 10.1080/09537100600931316. [DOI] [PubMed] [Google Scholar]
- 4.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332(6163):459–461. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 5.Chabrolle C, Tosca L, Ramé C, Lecomte P, Royère D, Dupont J. Adiponectin increases insulin-like growth factor I-induced progesterone and estradiol secretion in human granulosa cells. Fertil Steril. 2009;92(6):1988–1996. doi: 10.1016/j.fertnstert.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Childs AJ, Kinnell HL, Collins CS, Hogg K, Bayne RA, Green SJ, et al. BMP signaling in the human fetal ovary is developmentally regulated and promotes primordial germ cell apoptosis. Stem Cells. 2010;28(8):1368–1378. doi: 10.1002/stem.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cioffi JA, Blerkom J, Antczak M, Shafer A, Wittmer S, Snodgrass HR. The expression of leptin and its receptors in pre-ovulatory human follicles. Mol Hum Reprod. 1997;3(6):467–472. doi: 10.1093/molehr/3.6.467. [DOI] [PubMed] [Google Scholar]
- 8.Dagogo-Jack S, Fanelli C, Paramore D, Brothers J, Landt M. Plasma leptin and insulin relationships in obese and nonobese humans. Diabetes. 1996;45(5):695–698. doi: 10.2337/diabetes.45.5.695. [DOI] [PubMed] [Google Scholar]
- 9.Placido G, Alviggi C, Clarizia R, Mollo A, Alviggi E, Strina I, et al. Intra-follicular leptin concentration as a predictive factor for in vitro oocyte fertilization in assisted reproductive techniques. J Endocrinol Invest. 2006;29(8):719–726. doi: 10.1007/BF03344182. [DOI] [PubMed] [Google Scholar]
- 10.Erickson GF, Fuqua L, Shimasaki S. Analysis of spatial and temporal expression patterns of bone morphogenetic protein family members in the rat uterus over the estrous cycle. J Endocrinol. 2004;182(2):203–217. doi: 10.1677/joe.0.1820203. [DOI] [PubMed] [Google Scholar]
- 11.Hall J, Roberts R, Vora N. Energy homoeostasis: the roles of adipose tissue-derived hormones, peptide YY and Ghrelin. Obes Facts. 2009;2(2):117–125. doi: 10.1159/000208517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karamouti M, Kollia P, Kallitsaris A, Vamvakopoulos N, Kollios G, Messinis IE. Growth hormone, insulin-like growth factor I, and leptin interaction in human cultured lutein granulosa cells steroidogenesis. Fertil Steril. 2008;90(4 Suppl):1444–1450. doi: 10.1016/j.fertnstert.2007.08.076. [DOI] [PubMed] [Google Scholar]
- 13.Kolaczynski JW, Nyce MR, Considine RV, Boden G, Nolan JJ, Henry R, et al. Acute and chronic effects of insulin on leptin production in humans: studies in vivo and in vitro. Diabetes. 1996;45(5):699–701. doi: 10.2337/diabetes.45.5.699. [DOI] [PubMed] [Google Scholar]
- 14.Labruna G, Pasanisi F, Nardelli C, Caso R, Vitale DF, Contaldo F, et al. High leptin/adiponectin ratio and serum triglycerides are associated with an “at-risk” phenotype in young severely obese patients. Obesity (Silver Spring, Md). 2010. doi:10.1038/oby.2010.309. [DOI] [PubMed]
- 15.Ledoux S, Campos DB, Lopes FL, Dobias-Goff M, Palin M-F, Murphy BD. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology. 2006;147(11):5178–5186. doi: 10.1210/en.2006-0679. [DOI] [PubMed] [Google Scholar]
- 16.Leung K-C, Xu A, Craig ME, Martin A, Lam KSL, O’sullivan AJ. Adiponectin isoform distribution in women–relationship to female sex steroids and insulin sensitivity. Metab Clin Exp. 2009;58(2):239–245. doi: 10.1016/j.metabol.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Mcelroy SL, Byrne JA, Chavez SL, Behr B, Hsueh AJ, Westphal LM, et al. Parthenogenic blastocysts derived from cumulus-free in vitro matured human oocytes. PLoS One. 2010;5(6):e10979. doi: 10.1371/journal.pone.0010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalakis KG, Segars JH. The role of adiponectin in reproduction: from polycystic ovary syndrome to assisted reproduction. Fertil Steril. 2010;94(6):1949–1957. doi: 10.1016/j.fertnstert.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards JS, Liu Z, Kawai T, Tabata K, Watanabe H, Suresh D, et al. Adiponectin and its receptors modulate granulosa cell and cumulus cell functions, fertility, and early embryo development in the mouse and human. Fertil Steril. 2012;98(2):471–479.e1. doi: 10.1016/j.fertnstert.2012.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson F, Whitehead JP. Adiponectin—it’s all about the modifications. Int J Biochem Cell Biol. 2010;42(6):785–788. doi: 10.1016/j.biocel.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Stefan N, Vozarova B, Funahashi T, Matsuzawa Y, Weyer C, Lindsay RS, et al. Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes. 2002;51(6):1884–1888. doi: 10.2337/diabetes.51.6.1884. [DOI] [PubMed] [Google Scholar]
- 23.Unkila-Kallio L, Andersson S, Koistinen HA, Karonen SL, Ylikorkala O, Tiitinen A. Leptin during assisted reproductive cycles: the effect of ovarian stimulation and of very early pregnancy. Hum Reprod. 2001;16(4):657–662. doi: 10.1093/humrep/16.4.657. [DOI] [PubMed] [Google Scholar]
- 24.Tol HTA, Vernooij JCM, Colenbrander B, Gutknecht D, Macklon NS, Roelen BAJ. Expression of leptin receptor mRNA in cumulus cells is correlated with expression of PTX3. Reprod Biomed Online. 2010;20(6):741–750. doi: 10.1016/j.rbmo.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Vardhana PA, Dicken C, Tortoriello DV, Chu M, Carmina E, Lobo RA. Increasing adiposity in normal ovulatory women affects adipocytokine expression in subcutaneous and visceral abdominal fat. Int J Gynecol Obstet. 2009;104(2):121–124. doi: 10.1016/j.ijgo.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Xita N, Papassotiriou I, Georgiou I, Vounatsou M, Margeli A, Tsatsoulis A. The adiponectin-to-leptin ratio in women with polycystic ovary syndrome: relation to insulin resistance and proinflammatory markers. Metab Clin Exp. 2007;56(6):766–771. doi: 10.1016/j.metabol.2007.01.008. [DOI] [PubMed] [Google Scholar]