Abstract
Purpose
Down-regulation with gonadodropin-releasing agonist (GnRH-a) protocol during IVF stimulation leads to a severe endogenous LH suppression, which may affect the follicular development. The aim of the study was to evaluate the effects of recombinant LH (r-LH) administration, during late follicular development stages, in recombinant FSH (r-FSH) stimulated cycles on follicular fluid (FF) parameters and on cumulus cell quality.
Methods
Twenty patients undergoing IVF were stimulated in a long GnRH agonist protocol with r-FSH alone or with r-LH supplementation when the leading follicle reached diameter of 14 mm. FF was collected at the time of oocyte retrieval from 32 follicles ≥ 18 mm. Serum FSH, LH, estradiol (E2), and progesterone (P4) were evaluated on the day of hCG administration. Intra-follicular E2, P4, AMH and TGF-β were assayed. Total RNA from 18 individual cumuli was isolated for gene expression analyses.
Results
R-LH increased FF P4 levels. FF TGF-β levels and PTGS2 and HAS2 expression in cumulus cells (CCs) positively correlated with increased P4 levels observed in FFs, while a negative correlation was found between P4 and AMH levels.
Conclusions
FF positive correlation between P4 and TGF-β levels and CC expression of PTGS2 and HAS2 suggest an association with a better follicle quality. In addition, our data suggest that late follicular phase r-LH supplementation leads to a more advanced stage of follicular maturation.
Keywords: Progesterone, Follicular fluid, GNRH agonist, Recombinant luteinizing hormone, Recombinant follicle stimulating hormone, Cumulus cells
Introduction
In natural cycles both LH and FSH play a fundamental and complementary role in the development of follicles and ovulation [7,25]. In fact, although pharmacological doses of FSH alone are capable of stimulating ovarian follicular development, LH is strictly necessary to achieve final follicular maturation and oocyte fertilization [4,11]. Standard stimulation regimens typically use exogenous FSH or human menopausal gonadotropin (hMG) in conjunction with gonadotropin-releasing hormone agonists (GnRH-a) to suppress endogenous gonadotropin release [50]. Down-regulation with GnRH-a in some normogonadotrophic women may result in a profound suppression of endogenous LH concentration impairing adequate E2 synthesis. The stimulation with recombinant FSH (r-FSH) alone, containing negligible quantity of LH, has been shown to interfere with optimal oocyte maturation [17,56,58], while providing LH activity in gonadotropin treatment after GnRH-a down-regulation, has been shown to induce a significant higher proportion of top-quality embryos [37].
Although LH can substitute for FSH in supporting the later stages of follicular growth [55] and can stimulate optimal follicle-oocyte maturation [33], its role in controlled ovarian stimulation (COS) is still controversial [29,44].
LH has an essential role both in ovarian steroid synthesis and ovulation. In the initial phases of follicle growth, LH receptors (LH-Rs) are present only on theca cells (TC). Later on, in the mid- to late-follicular phases, FSH-stimulated granulosa cells (GC) acquire LH-Rs, and become capable to respond to LH stimulation, which drives final follicular maturation [16,32,55,61].
In prospective randomized trials performed in non selected patients, recombinant LH (r-LH) supplementation is not associated with better outcome in IVF [5,18]. However, in women above 35 years of age, poor responders, or with more severe pituitary GnRH-a suppression, stimulation with r-LH in the mid/late-follicular phase could be associated with improved results [6,12,24,34,37,40,41,47]. In a recent meta-analysis study it has been suggested that the supplementation with r-LH in patients of advanced reproductive age stimulated with r-FSH, may improve implantation and clinical pregnancy without affecting metaphase II (Met II) yield [31].
Even though live birth rate represents the most important outcome when evaluating the different protocols of COS, the possibility to evaluate the quality of follicles and, as a consequence, of the enclosed oocytes holds great importance. Exploration of intrafollicular levels and ratios of E2 and P4, as well as the levels of TGF-β and AMH would provide an additional insight into the impact of stimulation with exogenous LH activity. In fact, different stimulation regimens cause several differences in the FF levels of hormones and peptides. The addition of LH/hCG-containing preparations, such as hMG, from the beginning of stimulation induces higher production of E2 and lower production of P4 respect to stimulation with FSH alone [52]. These data support the notion that a low dosage of LH from the beginning of stimulation might be necessary to support the production of androgens by TCs and as a consequence the production of E2 by GCs. Moreover, differences have also been found in the levels of VEGF, inhibin A and B [52] and TGF-β [28]. It is noteworthy that AMH and TGF-β are differentially regulated in the preovulatory follicle. Two forms of TGFs are produced in the ovary: TCs produce TGF-α and -β, while GCs produce only TGF-β [45]. In the human ovary, TGF-α stimulates GCs growth while TGF-β inhibits their proliferation [38]. TGF-β which is stimulated by IGF-I and inhibin [32], suppresses the enzyme converting P4 to androgens and stimulates P450scc, thus leading to an increased P4 production [26,30]. TGF-β has been detected in human ovarian FFs [45], and its expression is increased in GCs from large preovulatory follicles [38]. Moreover, the levels of TGF-β in the FFs were found to correlate positively with that of E2 at egg retrieval and with the number of fertilized oocytes [27]. Conversely, AMH expression in ovarian follicles increases significantly from the primary through the early antral stages of folliculogenesis [14,57] and declines during the final follicular maturation process [3,22,23].
After the LH surge CCs secrete a muco-elastic matrix enriched in hyaluronic acid and undergo a dramatic expansion [48]. This process require gonadotropin stimulation as well as the presence of factors produced by the oocyte itself such as growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15). The expanded cumulus provides the proper environment for successful fertilization while reduced expansion severely impairs fertility [49]. The induction of genes such as hyaluronic acid synthase 2 (HAS2), gremlin 1 (GREM1) and cyclooxygenase 2 (PTGS2) which play essential roles in this process requires the presence of an healthy oocyte. Therefore the expression of these genes in CCs might reflect oocyte quality and give a direct assessment of fertility potential of oocyte [9].
The present study aims to evaluate the effects of r-LH supplementation to r-FSH in the late stages of follicle development on several parameters of FF associated with follicle quality, such as the profile of P4 and E2, and the levels of TGF-β and AMH. Moreover, we performed a preliminary study by examining the levels of expression of hyaluronic acid synthase 2 (HAS2), gremlin 1 (GREM1) and cyclooxygenase 2 (PTGS2) in cumulus cells.
Materials and methods
Patients
Follicular fluids (FFs) were collected from 32 follicles ≥ 18 mm retrieved from 20 patients undergoing IVF treatment at the C.I.P.A. Rome, Italy. Cumulus cells (CCs) were obtained from 18 out of the 32 follicular fluids. Informed consent was obtained from all patients. The study population consisted of ICSI patients with major indications for ART such as tubal infertility or unexplained infertility and/or partners with semen abnormalities. Average patient age was 33 years (range 31–42). All patients were with regular menstrual cycles of 25–32 days, presumed to be ovulatory. The early follicular phase serum levels of FSH were within normal limits (1–12 IU/l). The antral follicle count, assessed by ultrasound before starting the stimulation protocol, was within 6 and 12 follicles. The body mass index was in the range 18–25 kg/m2. Patients with polycystic ovary syndrome and patients with endometriosis were not included in the study. The main clinical characteristics of the patients are shown in Table 1.
Table 1.
Patient characteristics
| Baseline parameters | ||
|---|---|---|
| Group A (r-FSH) | Group B (r-FSH+r-LH) | |
| N° of patients | 11 | 9 |
| N° of follicles | 5 ± 0.4 | 5.2 ± 0.4 |
| N° of oocytes | 4.2 ± 0.3 | 4.1 ± 0.3 |
| Mean age (y) | 32.3 ± 0.3 | 34.1 ± 1.4 |
| Body mass index (BMI) (Kg/m2) | 22.6 ± 0.97 | 22.7 ± 0.75 |
| Serum LH (IU/L) after down-regulation | 0.42 ± 0.04 | 0.85 ± 0.08 |
| Serum values on hCG day | ||
| LH (IU/L) | 0.4 ± 0.06 | 0.96 ± 0.15** |
| FSH (IU/L) | 19.4 ± 0.4 | 18.5 ± 6.4 |
| E2 (μg/L) | 646 ± 124 | 1202 ± 157* |
| P4 (μg/L) | 0.53 ± 0.04 | 0.66 ± 0.11 |
Values are mean ± SEM. *P < 0.05, **P < 0.01 vs. Group A (r-FSH)
Patients underwent controlled ovarian stimulation following down-regulation with GnRH agonist (GnRH-a) in a long protocol. Pituitary suppression was carried out with 0.2 mL/d Buserelin, a GnRH-a (Suprefact injection, Sanofi-aventis SpA, Milano, Italy) starting on day 21 of the previous cycle. At this time, age-matched patients were randomized in two groups on one-to-one basis.
The patients were stimulated with r-FSH (Gonal-F, Merck Serono S.A., Geneva, Switzerland) alone (Group A) or with r-LH (Luveris; Merck Serono S.A., Geneva, Switzerland) supplementation (Group B) administered when the leading follicles reached 14 mm of diameter. The starting dose of r-FSH was 150 UI for the first 5 days, followed by individual adjustment according to the patient’s follicular response.
The doses of gonadotropins were individualized according to serum E2 levels and transvaginal ultrasound measurements of the developing follicles (r-FSH 100–225 IU/day and r-LH 75 IU/day up to 3 days).
Ovulation was induced with 10,000 IU hCG (Gonasi 5000 H, IBSA Farmaceutici Italia Srl, Lodi, Italy) when the leading follicles reached a diameter of 18–20 mm measured by transvaginal ultrasound and when E2 levels indicated a satisfactory follicular response. Transvaginal oocyte retrieval assisted by ultrasound monitoring was performed approximately 36 h after hCG.
Serum and follicular fluid collection
Blood samples were obtained on the day of hCG administration.
Follicular fluids were aspirated from individual follicles with a diameter of 18–22 mm. Only the initial clear fluid from aspirated follicles was saved for analysis. The fluid from single follicles were centrifuged at 1000 × g for 10 min to remove GCs, and stored at −80 °C for subsequent analysis. FFs contaminated with blood were excluded. Only FFs from follicles containing a Met II oocyte were further processed.
Hormonal measurements in serum and FFs
Serum E2, P4, LH and FSH and intra-follicular E2, P4 concentrations were determined using fluorescence enzymatic dosage techniques (ELFA) (Vidas®-BioMèrieux Italia SpA, Bagno a Ripoli (FI) Italia). The assay detection limits were 10 pg/mL, 0.15 ng/mL, 0.1 mIU/mL and 0.1 mIU/mL for E2, P4, FSH and LH, respectively. The intra- and inter-assay coefficients of variation were 4.5–6.3 % for E2, 4.3–5.4 % for P4, 4.2–4.7 % for FSH and 4.1–4.5 % for LH.
FF samples for E2 and P4 assays were diluted 1:1000 v/v in steroid-free serum just before measurement.
Assay for AMH
FF AMH levels were determined using an enzyme-immunometric assay Active MIS/AMH ELISA kit, DSL-10-14400 (Diagnostic Systems Laboratories Inc., TX, USA), according to the manufacturer’s instructions. The limit of detection was 0.01 ng/ml and the intra- and inter-assay coefficients of variations were 3.4 % and 6.5 %.
Assay for TGF-β
Active TGF-β was assayed by adding 100 μl of FF, diluted 1:10 v/v with DMEM, for 16 h to sub-confluent mink lung epithelial cells (MLECs) that were stably transfected with a PA inhibitor-1 promoter luciferase construct [1]. At the end of culture, the cells were washed twice with phosphate-buffered saline (PBS) and lysed in 60 μl of lysis buffer (Promega, Madison, WI). Cell extracts were assayed for luciferase activity with an assay kit obtained from Promega, using a Berthold luminometer. Parallel MLECs were incubated with known increasing concentrations of TGF-β to obtain a standard curve.
Cumulus cell collection
Oocyte-cumulus complexes (OCCs) were retrieved 36 h after hCG and separated from follicular fluid. After 2 h at 37 °C the oocytes were freed from cumulus cells by treatment with hyaluronidase (80 IU/ml, Medicult, Jyllinge, Denmark). Cells from single OCCs were washed in PBS and then subjected to centrifugation at 300 g for 15 min. The supernatant was removed and the pellet was immediately resuspended in 200 μl lysis buffer for RNA extraction and stored at −80 °C until used.
RNA extraction and reverse transcription
Total RNA from CCs was isolated by a silica gel-based membrane spin column (RNeasy Kit, Qiagen S.p.A., Milan, Italy), according to the manufacturer’s instructions. The purity and integrity of the RNA was checked spectroscopically and by gel electrophoresis. Total RNA (0.4–0.6 μg/cumulus) from each cumulus was reverse-transcribed in a final volume of 20 μl, using the M-MLV Reverse Transcriptase kit (Invitrogen, Milan, Italy).
Multiplex Polymerase Chain Reaction (PCR)
To determine the presence of the specific transcripts, duplex-PCR was performed (one gene of interest and the houskeeping gene in each sample). The reactions were carried out using a Multiplex PCR Kit (Qiagen S.p.A,. Milan, Italy) according to the manufacturer’s instructions. The chosen primer sequences are the following: PTGS2 Fw: 5’-TTCTGAAACCCACTCCAAACAC-3’ and Rw: 5’-CCCTCGCTTATGATCTGTCTTG-3’ (413 bp, NM_000963.2), HAS2 Fw: 5’-ATGACCTACGAAGCGATTATCACTGGATTC-3’ and Rw: 5’-AGAACTGTCTGTTTGGATTCTGAAAATGGC-3’ (410 bp, NM_005328.2), GREM1 Fw: 5’-GGCAGCAGTAATCTTCTTTTAGGAG-3’ and Rw: 5’-AACTTACAGACTTCCAGGATGTGTG-3’ (434 bp, NM_013372.6). The housekeeping geneβ-actin Fw: 5’-AAAATCTGGCACACCTTCTAC-3’ and Rw: 5’-AGGAGGAGCAATGATCTTGATCTTC-3’ (750 bp, NM_001101.3) was used as an internal control. Each primer pair was previously tested alone for specific amplification. For each sample, 10 μl of the PCR product was subjected to electrophoresis on a 2 % agarose gel and stained with ethidium bromide. The densitometric evaluation of the bands was performed with AIDA software (Advanced Image Data Analyzer, 2.11 Raytest GmbH, Straubenhartd, Germany). The relative mRNA levels were normalized against the expression of the housekeeping gene. The controls for DNA contamination were performed with gene-specific primers on RNA without reverse transcriptase treatment (data not shown).
Statistical analysis
Data are expressed as the mean ± SEM. The statistical analysis on each variable was performed using ANOVA followed by the Tukey-Kramer test for comparisons of multiple groups or two-tailed t test when comparing data derived from two groups. For assessment of correlations, Spearman’s rank-order correlation coefficients (rs) and their probability (P) levels were calculated. Values with P < 0.05 were considered statistically significant. All analyses were performed using Graphpad Instat for Windows (Graphpad Software, San Diego, CA, USA)
Results
Estradiol and Progesterone in the follicular fluid
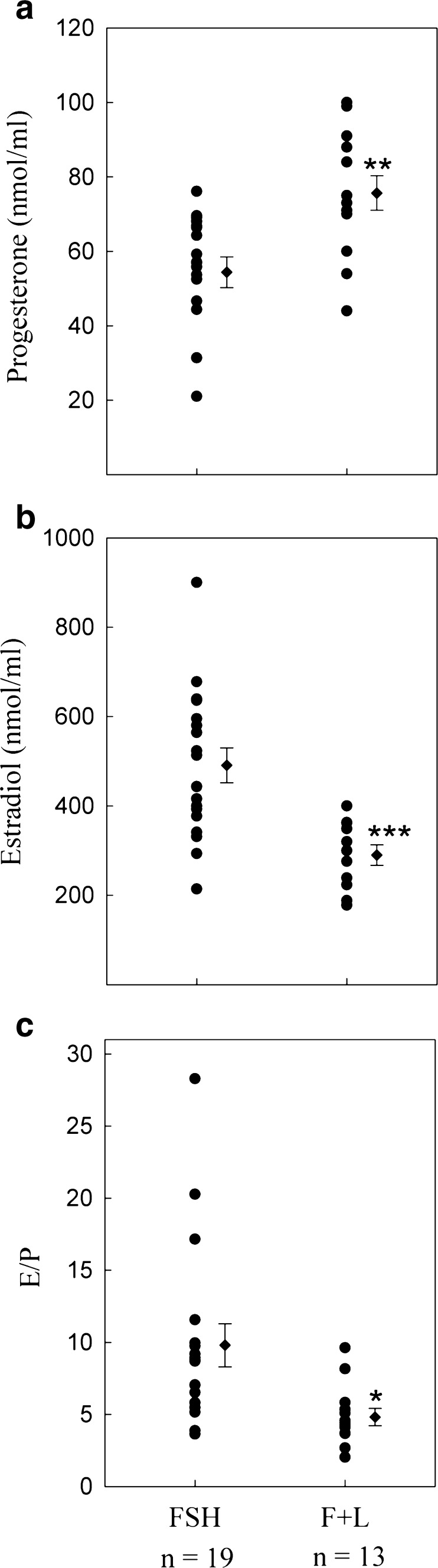
Figure 1a and b show the mean values of FF P4 and E2 concentrations for each of the treatment groups. P4 and E2 was measured in 19 FFs from patients treated with r-FSH alone, and in 13 FFs from patients treated with r-FSH+rLH. The concentrations of P4 and E2 in FF were statistically different among the two groups. In the group treated with r-FSH+r-LH, we observed higher levels of P4 compared with the FSH only group, and significantly lower levels of E2.
Fig. 1.
a Progesterone and b Estradiol levels in FFs obtained from single follicles at oocyte retrieval after stimulation with r-FSH (FSH), or r-FSH plus r-LH administered in the late follicular phase (F+L). c ratio of estradiol/progesterone (E/P). Each point represents the value obtained from a single FF. Dots on the rigth represent the mean ± S.E.M. *P < 0.05; **P < 0.01; ***P < 0.001. n = follicle numbers
As shown in Fig. 1c, the stimulation with r-LH in the r-FSH treated patients caused a decrease in the ratio of E2/P4.
TGF-β and AMH in the follicular fluid
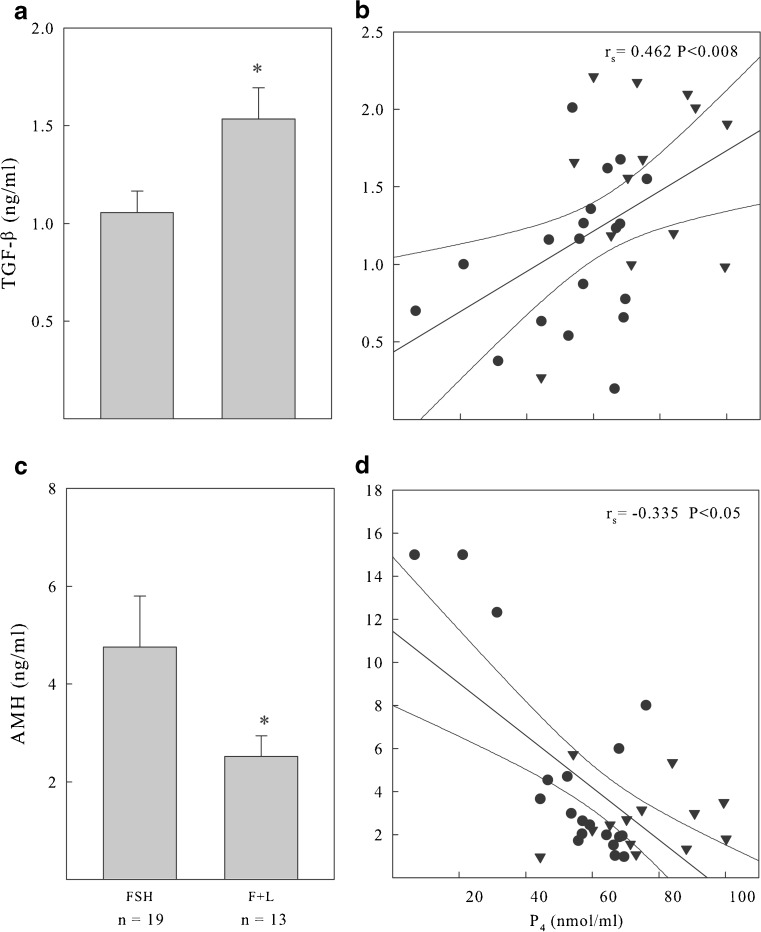
The levels of TGF-β and AMH in the FF from the same individual follicles analyzed above are shown in Fig. 2a and c, respectively. The amount of active TGF-β in the FF obtained at oocyte pick-up was significantly higher in the r-FSH+r-LH treated patients than in those treated with r-FSH alone. Opposite results were obtained when we measured the levels of AMH, as higher values in the r-FSH treated patients and lower values in those treated with r-FSH+r-LH were recorded.
Fig. 2.
a TGF-β and c AMH levels in FFs. FFs were collected from single follicles at oocyte retrieval after stimulation with r-FSH (FSH; n = 19), or r-FSH plus r-LH administered in the late follicular phase (F+L; n = 13). Relationships between TGF-β (b), AMH (d) and Progesterone (P4) levels in FFs. There is a positive and statistically significant relationship between TGF-β and follicular P4 levels while there is a negative and statistically significant relationship between AMH and P4. ● r-FSH ▼ r-FSH + r-LH; *P < 0.05
Correlation analysis of TGF-β AMH and Progesterone
Since TGF-β has been shown to stimulate steroidogenic genes such as P450scc necessary for P4 production, we compared active TGF-β and P4 levels in FFs. The levels of TGF-β correlated positively with the levels of P4 (Fig. 2b), irrespective of the stimulation used. At the same time, since AMH expression decreased after hCG, we also examined its relationship with P4 in FFs. We found that the levels of AMH were inversely correlated with the levels of P4 (Fig. 2d).
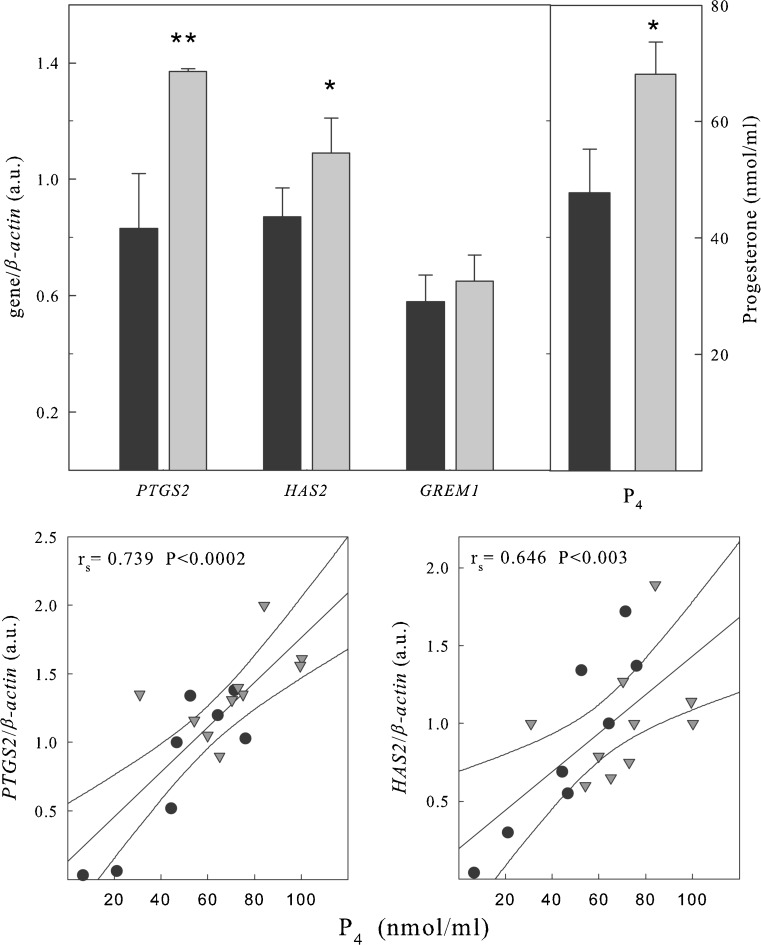
Expression of PTGS2, HAS2, and GREM1 in cumulus cells
The expression of genes indicating CC maturation was analyzed by multiplex RT-PCR. Only CCs from Met II oocytes were considered in this study. Eight cumuli were obtained from five patients stimulated with r-FSH alone and 10 from 8 patients further primed with r-LH when the leading follicles reached a diameter of 18–20 mm. As shown in Fig. 3, stimulation with LH increased the expression of PTGS2 and HAS2, while it had no significant effect on GREM1. When gene expression was correlated to P4 levels in the FF, gene expression of PTGS2 and HAS2 positively correlated with the levels of P4 in the FF, irrespective of the stimulation regimen adopted.
Fig. 3.
Upper panel: relative expression of PTGS2, HAS2 and GREM1 obtained by multiplex-PCR. Total RNA was extracted from CCs obtained from single follicles and subjected to multiplex RT-PCR using primers for PTGS2, HAS2 and GREM1, as well as for β-actin used as internal control. An aliquot of each PCR product was electrophoresed onto 2 % agarose gel and stained with ethidium bromide. Expression levels were quantified by densitometric evaluation of the bands with a chemiluminescence detection system (raytest) and values were normalised by their respective β-actin values and represent the mean ± S.E.M. In the right panel is represented the mean ± S.E.M. of the levels of P4 in the FFs corresponding to the CCs examined. *P < 0.05; **P < 0.01 vs. respective FSH. Lower panels: relationship between PTGS2 and HAS2 expression in CCs and the progesterone (P4) levels in the corresponding FFs. There is a positive and statistically significant relationship between PTGS2 and HAS2 and follicular P4 levels ● r-FSH (n = 8)▼ r-FSH+r-LH (n = 10)
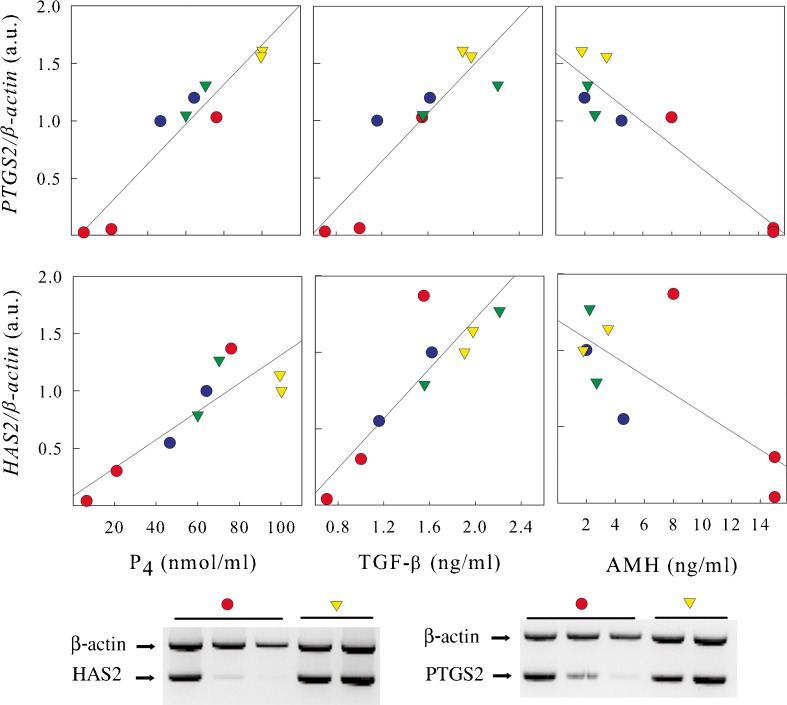
Two patients in each group had more than 1 cumulus examined. In order to correlate the responses obtained from different cumuli derived from the same patient we correlated mRNA levels of PTGS2 and HAS2 with the levels of P4, TGF-β and AMH found in the corresponding FFs. The number of patients is relatively small however, it was interesting that when we correlated the expression of PTGS2 and HAS2 with P4, TGF-β and AMH the same positive or negative correlation observed for the entire group was observed in CCs coming from single patients (Fig. 4).
Fig. 4.
Patients with more then 1 follicle retrieved. (Upper panel) Relationship between PTGS2 and HAS2 expression in CCs and the levels of progesterone (P4), TGF-β and AMH in the corresponding FFs. Two patients (5 follicles) treated with r-FSH (●), and two patients (4 follicles) treated with r-FSH+r-LH (▼). Symbols with the same colour belong to the same patient. The same positive or negative correlation observed for the entire group was observed in CCs coming from single patients. (Lower panel) Representative images of multiplex RT-PCR from one patient of the r-FSH group (● red) and one patient of the r-FSH+r-LH group (▼ yellow)
Discussion
In this study, we analyzed the levels of hormones and growth factors in FFs from individual follicles of patients treated, after GnRH-a down regulation, with r-FSH alone or followed by r-LH when the leading follicles reached 14 mm of diameter with the aim to verify possible benefits of stimulation with r-LH at late stages of follicular development in patients undergoing r-FSH induction.
The administration of r-LH to the r-FSH patients in the late phase of follicular development increased the levels of P4 and decreased the levels of E2 present in the FFs.
These data are in apparent contrast with data present in the literature. In fact, it has been shown that patients stimulated with hMG, presenting LH activity, show lower levels of P4 and higher levels of E2 compared to those stimulated with r-FSH alone [52]
However, this apparent discrepancy can be explained by the fact that, in the case of hMG stimulation, when growing follicles are exposed to the LH activity present in the hMG at the beginning of stimulation, when only TCs respond to LH, there is an increase in androgen production that, in turn, stimulates estrogen synthesis. On the contrary, when the addition of LH occurs in the late phase of follicular development, LH-Rs are already present on GCs that can now respond to LH and initiate luteinization with an increased production of P4 and decreased E2.
The levels of P4 in the serum during ovarian stimulation have been widely discussed. Several groups have shown that premature P4 elevation negatively affects the outcome of in vitro fertilization [19,51]. But also, the positive correlation between P4 and the number of mature follicles observed in the late follicular phase [21], suggests that increased P4 levels, at the correct time, are indicative of healthy follicular development. It has been shown that high levels of P4 are associated with higher pregnancy rate in donor oocyte IVF programs [35]. Therefore, the decrease in pregnancy rate observed in relation to high levels of P4 might reflect an impaired endometrial receptivity to embryo implantation instead of poor oocyte quality [35].
In our r-FSH+r-LH group of patients, we found high levels of P4 in FFs. However, these high hormone levels in the FFs were not correlated to the levels of P4 in serum. In fact, none of our patients had serum values of P4 > 0.9 μg/L (Table 1) on the day of oocyte retrieval, value that is reportedly associated with decreased pregnancy rates in IVF/ET cycles [19,51]. Moreover, the fact that, in accord with published data [24], we found similar levels of P4 in the two groups on the day of hCG suggests that LH administration in the late follicular phase does not cause early luteinization.
In order to evaluate the stage of follicle maturation we investigated the levels of two molecules present in the FF, namely TGF-β and AMH.
The administration of r-LH at later stages of folliculogenesis significantly increased TGF-β and decreased AMH levels.
Fried et al. [28], observed lower levels of TGF-β in the FFs of patients treated with hMG compared to the FFs of patients stimulated with r-FSH alone. However, it has been demonstrated in animal models that LH inhibits TGF-β secretion by rat and porcine TCs in culture [39,42]. Therefore, the LH activity present in hMG may affect TGF-β secretion by human TCs without affecting GCs, which at that specific time are unable to respond to LH. In our patients conversely, r-LH administered in the late follicular development was able to directly stimulate GCs to increase the production of TGF-β. Therefore, the increased P4 levels observed in the FFs of our patients treated with r-FSH+r-LH might be induced by the higher levels of TGF-β.
As an additional parameter to evaluate the degree of follicular maturation we measured the levels of AMH in the FFs of the different patients. We found statistically lower levels of AMH in the patients treated with r-FSH+r-LH respect to the r-FSH-only group. It has been demonstrated that AMH expression increases significantly from the primary through the early antral stages of folliculogenesis [13,57] and declines during the final follicular maturation process, suggesting a negative effect of follicle luteinization on the production of AMH in GCs [3,22,23]. Moreover, it has been shown a negative relationship between human FF AMH and P4 at the time of egg retrieval, suggesting an inverse correlation between a more advanced process of luteinization and ability of the follicle to secrete AMH [20].
Interestingly, when the values of TGF-β or AMH in the FFs were correlated with the levels of P4, we found that, irrespective of the stimulation used for our patients, there was a negative correlation between AMH and P4 and a positive correlation between TGF-β and P4 levels.
Therefore, our data suggest that these higher levels of P4 are indicative of more advanced follicle maturation.
It has been shown that paracrine and low molecular weight factors establish an actual dialogue between oocyte and cumulus cells [8]. Oocyte quality is assured by LH-driven nutritional and informational signals emanating in cumulus cells [2,36] and, at the same time, oocyte-derived factors influence GC functions such as cumulus expansion, apoptosis, carbohydrate metabolism and steroidogenesis [53,54] influencing their own microenvironment. Oocyte secretes GDF9, a TGF-β superfamily member that is required for normal folliculogenesis in mouse [15,59]. By secretion of GDF9 the oocyte directly influences CCs and controls an appropriate environment for its own correct maturation. Several genes downstream of GDF9 have been correlated with oocyte quality. Among these HAS2, GREM1 and PTSG2 [10,43,60]. Therefore, gene expression in cumulus cells can be a useful predictor of oocyte quality. We analyzed the expression of PTSG2, HAS2 and GREM1 in a total of 18 cumuli, 8 obtained from 5 patients treated with r-FSH alone and 10 obtained from 8 patients treated with rFSH followed by r-LH stimulation. Our preliminary results show that LH stimulation further increased the expression of HAS2 and PTSG2 whereas it did not affect GREM1 expression. Even more interestingly there was a positive correlation between levels of P4 in the FF and the expression levels of HAS2 and PTSG2
Taken together, our data suggest that the supplementation with r-LH in the late follicular phase leads to a more advanced stage of follicular maturation at least in those patients with low levels of LH after pituitary GnRH-a suppression.
These data are further supported by the fact that it has been shown that supplementation with r-LH during the late follicular phase significantly reduced the number of immature oocytes collected after pick-up, while it increased the number of embryos transferred [46] and reduced CC apoptosis [47].
Acknowledgments
MLECs were kindly provided by Dr.D.B. Rifkin, New York University School of Medicine, New York. We thank Dr. Giovanni Ruvolo, Centro di Biologia della Riproduzione, Palermo, Italy, for isolating cumulus cells; Dr. Paola Canipari for reviewing the English in the manuscript.
This study was supported by “La Sapienza” University of Rome Ateneo Federato 2009–2010 to R.C.
Footnotes
Marzia Barberi and Beatrice Ermini contributed equally to this work Sandra Cecconi and Rita Canipari contributed equally as co-senior authors
Capsule LH supplementation to an IVF stimulation with r-FSH leads to a more advanced stage of follicular maturation in the study population which presented normal or low LH levels after pituitary GnRH-a suppression.
References
- 1.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 2.Assou S, Haouzi D, Mahmoud K, Aouacheria A, Guillemin Y, Pantesco V, et al. A non-invasive test for assessing embryo potential by gene expression profiles of human cumulus cells: a proof of concept study. Mol Hum Reprod. 2008;14:711–719. doi: 10.1093/molehr/gan067. [DOI] [PubMed] [Google Scholar]
- 3.Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, Leeuwen EC, Themmen AP, et al. Anti-mullerian hormone and anti-mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136:4951–4962. doi: 10.1210/en.136.11.4951. [DOI] [PubMed] [Google Scholar]
- 4.Balasch J, Miro F, Burzaco I, Casamitjana R, Civico S, Ballesca JL, et al. The role of luteinizing hormone in human follicle development and oocyte fertility: evidence from in-vitro fertilization in a woman with long-standing hypogonadotrophic hypogonadism and using recombinant human follicle stimulating hormone. Hum Reprod. 1995;10:1678–1683. doi: 10.1093/oxfordjournals.humrep.a136154. [DOI] [PubMed] [Google Scholar]
- 5.Barrenetxea G, Agirregoikoa JA, Jimenez MR, Larruzea AL, Ganzabal T, Carbonero K. Ovarian response and pregnancy outcome in poor-responder women: a randomized controlled trial on the effect of luteinizing hormone supplementation on in vitro fertilization cycles. Fertil Steril. 2008;89:546–553. doi: 10.1016/j.fertnstert.2007.03.088. [DOI] [PubMed] [Google Scholar]
- 6.Bosch E, Vidal C, Labarta E, Simon C, Remohi J, Pellicer A. Highly purified hMG versus recombinant FSH in ovarian hyperstimulation with GnRH antagonists-a randomized study. Hum Reprod. 2008;23:2346–2351. doi: 10.1093/humrep/den220. [DOI] [PubMed] [Google Scholar]
- 7.Caglar GS, Asimakopoulos B, Nikolettos N, Diedrich K, Al-Hasani S. Recombinant LH in ovarian stimulation. Reprod Biomed Online. 2005;10:774–785. doi: 10.1016/S1472-6483(10)61123-6. [DOI] [PubMed] [Google Scholar]
- 8.Canipari R. Oocyte-granulosa cell interactions. Hum Reprod Update. 2000;6:279–289. doi: 10.1093/humupd/6.3.279. [DOI] [PubMed] [Google Scholar]
- 9.Canipari R, Cellini V, Cecconi S. The ovary feels fine when paracrine and autocrine networks cooperate with gonadotropins in the regulation of folliculogenesis. Curr Pharm Des. 2012;18:245–255. doi: 10.2174/138161212799040411. [DOI] [PubMed] [Google Scholar]
- 10.Cillo F, Brevini TA, Antonini S, Paffoni A, Ragni G, Gandolfi F. Association between human oocyte developmental competence and expression levels of some cumulus genes. Reproduction. 2007;134:645–650. doi: 10.1530/REP-07-0182. [DOI] [PubMed] [Google Scholar]
- 11.Couzinet B, Lestrat N, Brailly S, Forest M, Schaison G. Stimulation of ovarian follicular maturation with pure follicle-stimulating hormone in women with gonadotropin deficiency. J Clin Endocrinol Metab. 1988;66:552–556. doi: 10.1210/jcem-66-3-552. [DOI] [PubMed] [Google Scholar]
- 12.Placido G, Alviggi C, Perino A, Strina I, Lisi F, Fasolino A, et al. Recombinant human LH supplementation versus recombinant human FSH (rFSH) step-up protocol during controlled ovarian stimulation in normogonadotrophic women with initial inadequate ovarian response to rFSH. A multicentre, prospective, randomized controlled trial. Hum Reprod. 2005;20:390–396. doi: 10.1093/humrep/deh625. [DOI] [PubMed] [Google Scholar]
- 13.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084. doi: 10.1210/en.143.3.1076. [DOI] [PubMed] [Google Scholar]
- 14.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124:601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 15.Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13:1035–1048. doi: 10.1210/me.13.6.1035. [DOI] [PubMed] [Google Scholar]
- 16.Erickson GF, Wang C, Hsueh AJ. FSH induction of functional LH receptors in granulosa cells cultured in a chemically defined medium. Nature. 1979;279:336–338. doi: 10.1038/279336a0. [DOI] [PubMed] [Google Scholar]
- 17.Esposito MA, Barnhart KT, Coutifaris C, Patrizio P. Role of periovulatory luteinizing hormone concentrations during assisted reproductive technology cycles stimulated exclusively with recombinant follicle-stimulating hormone. Fertil Steril. 2001;75:519–524. doi: 10.1016/S0015-0282(00)01745-3. [DOI] [PubMed] [Google Scholar]
- 18.Fabregues F, Creus M, Penarrubia J, Manau D, Vanrell JA, Balasch J. Effects of recombinant human luteinizing hormone supplementation on ovarian stimulation and the implantation rate in down-regulated women of advanced reproductive age. Fertil Steril. 2006;85:925–931. doi: 10.1016/j.fertnstert.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 19.Fanchin R, Ziegler D, Taieb J, Hazout A, Frydman R. Premature elevation of plasma progesterone alters pregnancy rates of in vitro fertilization and embryo transfer. Fertil Steril. 1993;59:1090–1094. doi: 10.1016/s0015-0282(16)55933-0. [DOI] [PubMed] [Google Scholar]
- 20.Fanchin R, Louafi N, Mendez Lozano DH, Frydman N, Frydman R, Taieb J. Per-follicle measurements indicate that anti-mullerian hormone secretion is modulated by the extent of follicular development and luteinization and may reflect qualitatively the ovarian follicular status. Fertil Steril. 2005;84:167–173. doi: 10.1016/j.fertnstert.2005.01.115. [DOI] [PubMed] [Google Scholar]
- 21.Fanchin R, Righini C, Olivennes F, Ferreira AL, Ziegler D, Frydman R. Consequences of premature progesterone elevation on the outcome of in vitro fertilization: insights into a controversy. Fertil Steril. 1997;68:799–805. doi: 10.1016/S0015-0282(97)00337-3. [DOI] [PubMed] [Google Scholar]
- 22.Fanchin R, Schonauer LM, Righini C, Frydman N, Frydman R, Taieb J. Serum anti-Mullerian hormone dynamics during controlled ovarian hyperstimulation. Hum Reprod. 2003;18:328–332. doi: 10.1093/humrep/deg043. [DOI] [PubMed] [Google Scholar]
- 23.Fanchin R, Schonauer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18:323–327. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 24.Ferraretti AP, Gianaroli L, Magli MC, D’angelo A, Farfalli V, Montanaro N. Exogenous luteinizing hormone in controlled ovarian hyperstimulation for assisted reproduction techniques. Fertil Steril. 2004;82:1521–1526. doi: 10.1016/j.fertnstert.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 25.Filicori M, Cognini GE. Roles and Novel Regimens of Luteinizing Hormone and Follicle-Stimulating Hormone in Ovulation Induction. J Clin Endocrinol Metab. 2001;86:1437–1441. doi: 10.1210/jc.86.4.1437. [DOI] [PubMed] [Google Scholar]
- 26.Fournet N, Weitsman SR, Zachow RJ, Magoffin DA. Transforming growth factor-beta inhibits ovarian 17 alpha-hydroxylase activity by a direct noncompetitive mechanism. Endocrinology. 1996;137:166–174. doi: 10.1210/en.137.1.166. [DOI] [PubMed] [Google Scholar]
- 27.Fried G, Wramsby H. Increase in transforming growth factor beta1 in ovarian follicular fluid following ovarian stimulation and in-vitro fertilization correlates to pregnancy. Hum Reprod. 1998;13:656–659. doi: 10.1093/humrep/13.3.656. [DOI] [PubMed] [Google Scholar]
- 28.Fried G, Wramsby H, Tally M. Transforming growth factor-beta1, insulin-like growth factors, and insulin-like growth factor binding proteins in ovarian follicular fluid are differentially regulated by the type of ovarian hyperstimulation used for in vitro fertilization. Fertil Steril. 1998;70:129–134. doi: 10.1016/S0015-0282(98)00105-8. [DOI] [PubMed] [Google Scholar]
- 29.Griesinger G, Schultze-Mosgau A, Dafopoulos K, Schroeder A, Schroer A, Otte S, et al. Recombinant luteinizing hormone supplementation to recombinant follicle-stimulating hormone induced ovarian hyperstimulation in the GnRH-antagonist multiple-dose protocol. Hum Reprod. 2005;20:1200–1206. doi: 10.1093/humrep/deh741. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez ER, Hurwitz A, Payne DW, Dharmarajan AM, Purchio AF, Adashi EY. Transforming growth factor-beta 1 inhibits ovarian androgen production: gene expression, cellular localization, mechanisms(s), and site(s) of action. Endocrinology. 1990;127:2804–2811. doi: 10.1210/endo-127-6-2804. [DOI] [PubMed] [Google Scholar]
- 31.Hill MJ, Levens ED, Levy G, Ryan ME, Csokmay JM, DeCherney AH, et al. The use of recombinant luteinizing hormone in patients undergoing assisted reproductive techniques with advanced reproductive age: a systematic review and meta-analysis. Fertil Steril. 2012;97:1108–1114. doi: 10.1016/j.fertnstert.2012.01.130. [DOI] [PubMed] [Google Scholar]
- 32.Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. 2001;179:39–46. doi: 10.1016/S0303-7207(01)00469-5. [DOI] [PubMed] [Google Scholar]
- 33.Hillier SG. Paracrine support of ovarian stimulation. Mol Hum Reprod. 2009;15:843–850. doi: 10.1093/molehr/gap086. [DOI] [PubMed] [Google Scholar]
- 34.Humaidan P, Bungum M, Bungum L, Yding AC. Effects of recombinant LH supplementation in women undergoing assisted reproduction with GnRH agonist down-regulation and stimulation with recombinant FSH: an opening study. Reprod Biomed Online. 2004;8:635–643. doi: 10.1016/S1472-6483(10)61643-4. [DOI] [PubMed] [Google Scholar]
- 35.Legro RS, Ary BA, Paulson RJ, Stanczyk FZ, Sauer MV. Premature luteinization as detected by elevated serum progesterone is associated with a higher pregnancy rate in donor oocyte in-vitro fertilization. Hum Reprod. 1993;8:1506–1511. doi: 10.1093/oxfordjournals.humrep.a138288. [DOI] [PubMed] [Google Scholar]
- 36.Li HK, Kuo TY, Yang HS, Chen LR, Li SS, Huang HW. Differential gene expression of bone morphogenetic protein 15 and growth differentiation factor 9 during in vitro maturation of porcine oocytes and early embryos. Anim Reprod Sci. 2008;103:312–322. doi: 10.1016/j.anireprosci.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Lisi F, Rinaldi L, Fishel S, Caserta D, Lisi R, Campbell A. Evaluation of two doses of recombinant luteinizing hormone supplementation in an unselected group of women undergoing follicular stimulation for in vitro fertilization. Fertil Steril. 2005;83:309–315. doi: 10.1016/j.fertnstert.2004.07.969. [DOI] [PubMed] [Google Scholar]
- 38.Lobb DK. Expression and actions of transforming growth factors during human follicular development. Fertil Steril. 2009;92:1080–1084. doi: 10.1016/j.fertnstert.2008.07.1736. [DOI] [PubMed] [Google Scholar]
- 39.Magoffin DA, Hubert-Leslie D, Zachow RJ. Estradiol-17 beta, insulin-like growth factor-I, and luteinizing hormone inhibit secretion of transforming growth factor beta by rat ovarian theca-interstitial cells. Biol Reprod. 1995;53:627–635. doi: 10.1095/biolreprod53.3.627. [DOI] [PubMed] [Google Scholar]
- 40.Marrs R, Meldrum D, Muasher S, Schoolcraft W, Werlin L, Kelly E. Randomized trial to compare the effect of recombinant human FSH (follitropin alfa) with or without recombinant human LH in women undergoing assisted reproduction treatment. Reprod Biomed Online. 2004;8:175–182. doi: 10.1016/S1472-6483(10)60513-5. [DOI] [PubMed] [Google Scholar]
- 41.Matorras R, Prieto B, Exposito A, Mendoza R, Crisol L, Herranz P, et al. Mid-follicular LH supplementation in women aged 35–39 years undergoing ICSI cycles: a randomized controlled study. Reprod Biomed Online. 2009;19:879–887. doi: 10.1016/j.rbmo.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 42.May JV, Turzcynski CJ, Ramos L, Mau YH. Differential involvement of protein kinase C in the regulation of transforming growth factor-beta (TGF-beta) secretion by porcine theca and granulosa cells in vitro. Endocrinology. 1995;136:1319–1322. doi: 10.1210/en.136.3.1319. [DOI] [PubMed] [Google Scholar]
- 43.McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE, et al. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. 2004;19:2869–2874. doi: 10.1093/humrep/deh535. [DOI] [PubMed] [Google Scholar]
- 44.Mochtar MH, Van d, V, Ziech M, van WM (2007) Recombinant Luteinizing Hormone (rLH) for controlled ovarian hyperstimulation in assisted reproductive cycles. Cochrane Database Syst Rev CD005070 [DOI] [PubMed]
- 45.Mulheron GW, Bossert NL, Lapp JA, Walmer DK, Schomberg DW. Human granulosa-luteal and cumulus cells express transforming growth factors-beta type 1 and type 2 mRNA. J Clin Endocrinol Metab. 1992;74:458–460. doi: 10.1210/jc.74.2.458. [DOI] [PubMed] [Google Scholar]
- 46.Pezzuto A, Ferrari B, Coppola F, Nardelli GB. LH supplementation in down-regulated women undergoing assisted reproduction with baseline low serum LH levels. Gynecol Endocrinol. 2010;26:118–124. doi: 10.3109/09513590903215516. [DOI] [PubMed] [Google Scholar]
- 47.Ruvolo G, Bosco L, Pane A, Morici G, Cittadini E, Roccheri MC. Lower apoptosis rate in human cumulus cells after administration of recombinant luteinizing hormone to women undergoing ovarian stimulation for in vitro fertilization procedures. Fertil Steril. 2007;87:542–546. doi: 10.1016/j.fertnstert.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 48.Salustri A, Camaioni A, D’Alessandris C. Endocrine and paracrine regulation of cumulus expansion. Zygote. 1996;4:313–315. doi: 10.1017/S0967199400003312. [DOI] [PubMed] [Google Scholar]
- 49.Salustri A, Garlanda C, Hirsch E, Acetis M, Maccagno A, Bottazzi B, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–1586. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- 50.Santos MA, Kuijk EW, Macklon NS. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction. 2010;139:23–34. doi: 10.1530/REP-09-0187. [DOI] [PubMed] [Google Scholar]
- 51.Silverberg KM, Burns WN, Olive DL, Riehl RM, Schenken RS. Serum progesterone levels predict success of in vitro fertilization/embryo transfer in patients stimulated with leuprolide acetate and human menopausal gonadotropins. J Clin Endocrinol Metab. 1991;73:797–803. doi: 10.1210/jcem-73-4-797. [DOI] [PubMed] [Google Scholar]
- 52.Smitz J, Andersen AN, Devroey P, Arce JC. Endocrine profile in serum and follicular fluid differs after ovarian stimulation with HP-hMG or recombinant FSH in IVF patients. Hum Reprod. 2007;22:676–687. doi: 10.1093/humrep/del445. [DOI] [PubMed] [Google Scholar]
- 53.Sugiura K, Pendola FL, Eppig JJ. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol. 2005;279:20–30. doi: 10.1016/j.ydbio.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 54.Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, et al. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134:2593–2603. doi: 10.1242/dev.006882. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan MW, Stewart-Akers A, Krasnow JS, Berga SL, Zeleznik AJ. Ovarian responses in women to recombinant follicle-stimulating hormone and luteinizing hormone (LH): a role for LH in the final stages of follicular maturation. J Clin Endocrinol Metab. 1999;84:228–232. doi: 10.1210/jc.84.1.228. [DOI] [PubMed] [Google Scholar]
- 56.Tesarik J, Mendoza C. Effects of exogenous LH administration during ovarian stimulation of pituitary down-regulated young oocyte donors on oocyte yield and developmental competence. Hum Reprod. 2002;17:3129–3137. doi: 10.1093/humrep/17.12.3129. [DOI] [PubMed] [Google Scholar]
- 57.Weenen C, Laven JS, Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 58.Westergaard LG, Laursen SB, Andersen CY. Increased risk of early pregnancy loss by profound suppression of luteinizing hormone during ovarian stimulation in normogonadotrophic women undergoing assist ed reproduction. Hum Reprod. 2000;15:1003–1008. doi: 10.1093/humrep/15.5.1003. [DOI] [PubMed] [Google Scholar]
- 59.Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/me.15.6.854. [DOI] [PubMed] [Google Scholar]
- 60.Yang SH, Son WY, Yoon SH, Ko Y, Lim JH. Correlation between in vitro maturation and expression of LH receptor in cumulus cells of the oocytes collected from PCOS patients in HCG-primed IVM cycles. Hum Reprod. 2005;20:2097–2103. doi: 10.1093/humrep/dei045. [DOI] [PubMed] [Google Scholar]
- 61.Zeleznik AJ. Follicle selection in primates: “many are called but few are chosen”. Biol Reprod. 2001;65:655–659. doi: 10.1095/biolreprod65.3.655. [DOI] [PubMed] [Google Scholar]