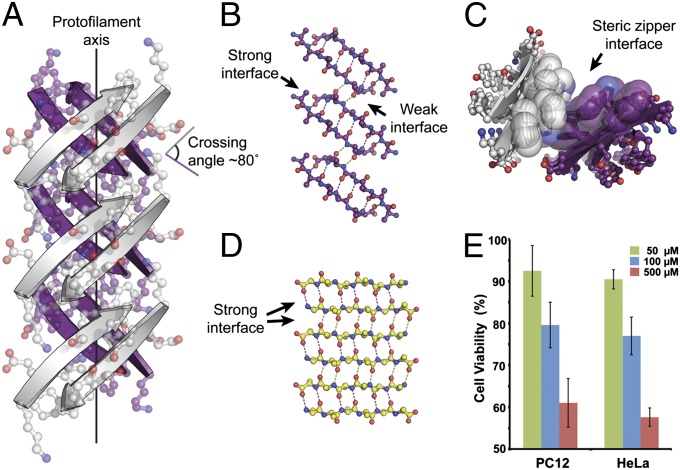
Fig. 1.
Crystal structure of out-of-register, antiparallel protofilament formed by the amyloidogenic peptide KDWSFY (β2m58–63) and its fibril toxicity. (A) View perpendicular to the protofilament axis. The two β-sheets are colored in purple and white. Side chains are shown in ball-and-stick representation. The protofilament axis is denoted by a black line. Note that the β-strands are antiparallel, out-of-register, and not perpendicular to the protofilament axis. The crossing angle between the β-strands of adjacent β-sheets is ∼80°. (B) The geometry of the back (purple) out-of-register β-sheet. For clarity, side chains are omitted. Hydrogen bonds are shown by dotted lines. (C) View down the protofilament axis showing the dry steric zipper interface between the pair of β-sheets. Side chains contributing to this interface are shown in space-filling representation. (D) The geometry of a single in-register antiparallel β-sheet from the KLVFFA crystal structure (Protein Data Bank ID code 2Y2A). (E) Assessment of cytotoxicity of KDWSFY (β2m58–63) fibril samples by MTT-based cell viability assay. KDWSFY (β2m58–63) fibrils show toxicity to both PC12 and HeLa cell lines in a dose-dependent manner. Error bars represent 1 SD (n = 4).