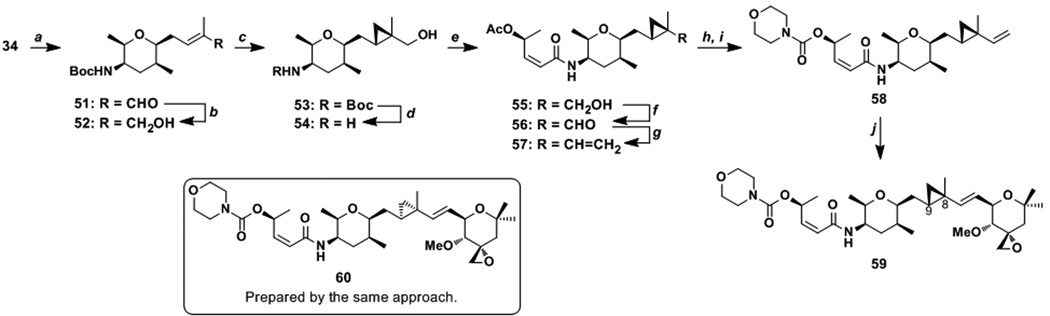
Scheme 7.
Preparation of cyclopropyl analogues. a Conditions: (a) methacrolein (20 equiv), Ru-1 (3 mol%), 23 °C, 84%. (b) DIBALH (2.0 equiv), CH2Cl2, −78 °C, 98%. (c) (S,S)-dioxaborolane (2.0 equiv), ZnEt2 (5.0 equiv), CH2I2 (7.0 equiv), CH2Cl2, 23 °C, 70%, d.r. = 89:11. (d) CH2Cl2/TFA (8:2), 0 to 23 °C, quant. (e) 19 (1.2 equiv), HATU (1.0 equiv), iPr2NEt (4.0 equiv), MeCN, 23 °C, 48%. (f) Dess-Martin periodinane (2.0 equiv), CH2Cl2, 0 °C, 70%. (g) Ph3PCH3Br (6.0 equiv), KOtBu (5.0 equiv), THF, 0 °C, 80%. (h) 1. K2CO3 (2.5 equiv), THF, 0 °C. (i) CDI (2.5 equiv), CH2Cl2, 23 °C; morpholine (5.0 equiv), 23 °C, 94% (over 2 steps). (j) 49 (1.7 equiv), Ru-1 (0.1 equiv), benzoquinone (0.2 equiv), toluene, 70 °C, 30%.