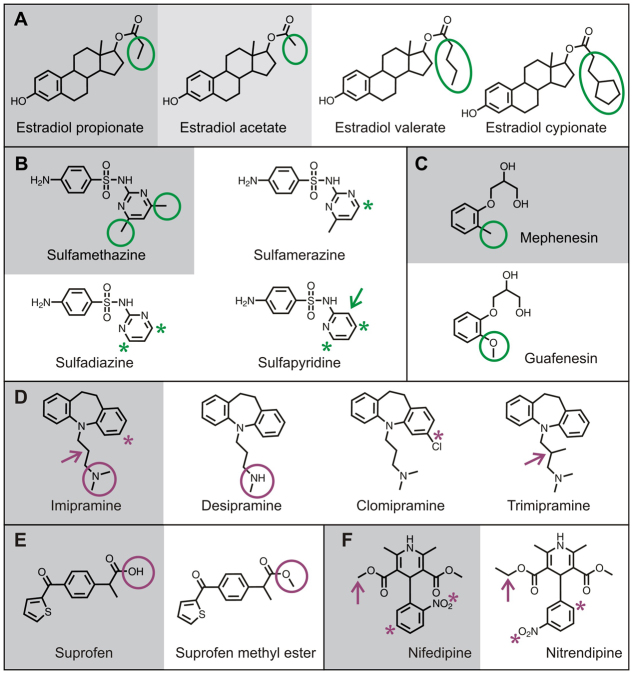
Fig. 6.
SAR analysis based on activity cliffs. (A–F) High-potency fascin-pathway modifiers (gray background) compared with structurally similar low-potency (light gray background) or inactive (white background) compounds. The highlighted substructures can be inferred to influence activity in the fascin bioassay. (A–C) Fascin-pathway enhancers (green highlights). (A) Estradiol propionate: the size of the side chain (oval) on the carboxy group on the sterol D ring is associated with activity, suggesting that in estradiol acetate the side chain is too small, whereas in estradiol valerate and cypionate, it is too large. (B) Sulfamethazine: activity is associated with dimethylation (circles) of the pyrimidine ring. Removal (asterisk) of one (sulfamerazine) or both (sulfadiazine and sulfapyridine) methyl groups is associated with loss of activity. (C) Mephenesin: replacement of the methyl group with a methoxy group in guaifenesin (circles) is associated with lack of activity. (D–F) Fascin-pathway blockers (magenta highlights). (D) Imipramine: in three other tricyclic compounds, single changes are associated with lack of activity. Desipramine is lacking a methyl group of the alkyl nitrogen (circles); clomipramine has a chloride on one of the aromatic rings (asterisks); trimipramine has a methyl substitution in the middle of the alkyl chain (arrows). (E) Suprofen: replacement of a hydrogen with a methyl group (circles) creates suprofen methyl ester, which is inactive. (F) Nifedipine: two changes, the position of the nitro group on the aromatic ring (asterisks) and addition of a methyl group (arrows), are associated with lack of activity of nitrendipine.