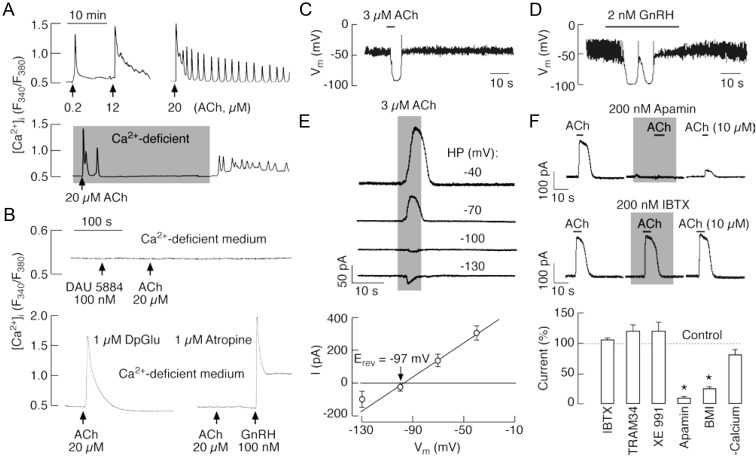
Fig. 4.
ACh-induced Ca2+ mobilization and activation of SK-type potassium channels in LβT2 gonadotrophs. A, Representative records of oscillatory and nonoscillatory [Ca2+]i responses induced by variable ACh concentrations (top) and extracellular calcium dependence of sustained but not early spiking (bottom). Gray area indicates period during which cells were exposed to Ca2+-deficient medium. B, Pharmacological identification of receptors responsible for ACh-induced Ca2+ mobilization. Top, Inhibition of Ca2+-mobilizing action of 20 μm ACh in cells bathed in Ca2+-deficient medium and treated with 100 nm DAU 5884, a M3-mAChR-specific inhibitor. Bottom, Insensitivity of ACh-induced Ca2+ response to DpGlu, a specific antagonist of GnRH receptor (left), and inhibition by atropine, a general mAChR blocker (right), in cells bathed in Ca2+-deficient medium. C and D, Current-clamp traces of ACh (C)- and GnRH (D)-induced transient hyperpolarization. E and F, Activation of KCa channels by ACh in LβT2 gonadotrophs. E, Effect of the holding potential (HP) on the amplitude of ACh-induced outward current: representative traces (top) and mean ± sem values (bottom). The reversal potential (Erev = −97 ± 5 mV, n = 3) was near to the calculated reversal potential for K+ ions. F, Sensitivity of ACh-induced current to KCa channel blockers. Top, Inhibition of ACh-induced current by apamin (200 nm), a small conductance KCa channel blocker, but not IBTX (200 nm), a highly specific blocker for KCa1.1 channels. Bottom, Summary histogram showing effects of removal of bath Ca2+ and addition of K+ channel blockers: apamin (200 nm), IBTX (100 nm), BMI (30 μm), TRAM 34 (1 μm), and Xe991 (1 μm), in cell bathed in Ca2+-containing medium. *, P < 0.01. LβT2 cells were voltage clamped at −40 mV using amphotericin-perforated whole-cell recording.