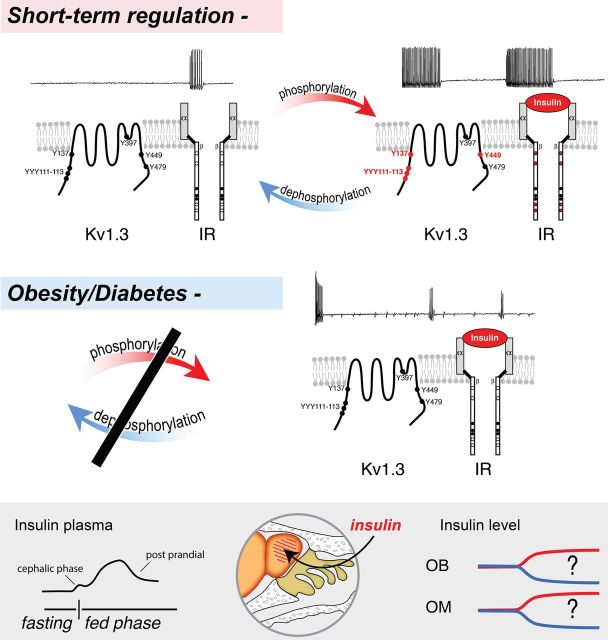
Figure 2.
A schematic representing the identity of a voltage-dependent potassium channel, Kv1.3, which is a substrate for insulin signaling in the OB. Kv1.3 is highly expressed in mitral neurons of the OB, where it carries a large proportion of voltage-gated outward currents (not shown) and mediates dampening of excitability by regulating action-potential timing and interspike interval (top left traces). Short-term regulation by insulin: Kv1.3 has multiple tyrosine (Y) residues (circles) that could serve as recognition motifs for tyrosine phosphorylation. Upon binding of the hormone insulin to the alpha subunits of the insulin receptor (IR) kinase (right side of schematic), multiple sites along the beta subunits (red circles) undergo autophosphorylation and the activated IR subsequently phosphorylates residues 111–113, 137, and 449 in the amino and carboxyl terminals of the Kv1.3 channel (red circles). As measured by recording under voltage-clamp conditions, phosphorylation causes a suppression of Kv1.3 via decreased open probability of the channel and via current clamp, it increases evoked action-potential frequency, spike train duration, and interspike interval while decreasing the pausing between spike clusters (top right traces). Obesity/Diabetes: Following diet-induced obesity (DIO) or chronic hyperglycemia of diabetes, the normal cycles of phosphorylation/dephosphorylation are imbalanced (black bar). Mice challenged with DIO exhibit mitral cell firing with irregular spike firing frequency, prominent spike adaptation, or partial amplitude. Application of acute insulin fails to increase spike frequency and Kv1.3 remains unphosphorylated (black circles)—both signs of insulin resistance. Bottom box: During natural cycles of feeding and fasting, olfactory physiology can be modulated through the rapid transport of insulin across the blood—brain barrier; but measured daily fluctuations in olfactory bulb (OB) and olfactory mucosal (OM) insulin are currently not reported.