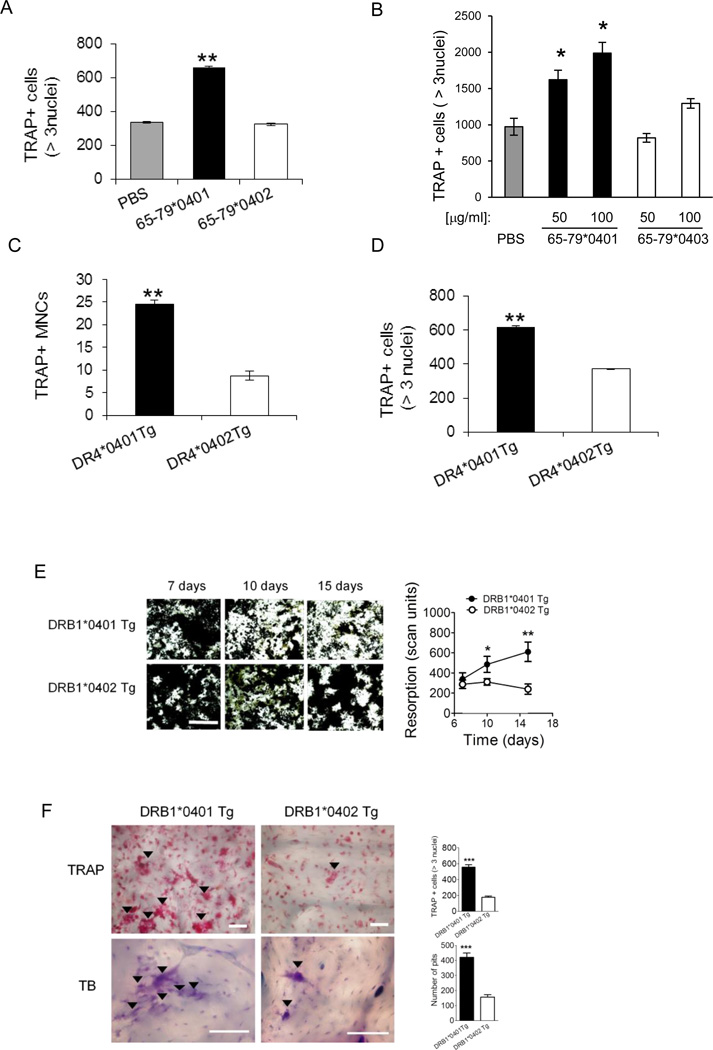
Figure 2. Osteoclastogenic effects of SE in primary cells.
(A) BMCs harvested from DBA/1 mice were cultured with M-CSF and RANKL in the presence of 50 µg/ml of the SE ligand 65-79*0401, a SE-negative control peptide 65-79*0402, or PBS for 6 days, and TRAP-positive cells counted. (B) OCs were differentiated from human PBMCs for 7 days in the presence or absence of the SE ligand 65-79*0401, or a SE-negative control peptide 65-79*0403. (C) BMCs from DR4*0401 Tg and DR4*0402 Tg mice in medium without growth factors were allowed to adhere to culture wells overnight and the number of TRAP-positive mononuclear pre-OC cells (MNCs) per well was determined. (D) BMCs from DR4*0401 Tg and DR4*0402 Tg mice were cultured for 6 days OC-differentiating conditions, and TRAP-positive multinucleated cells were counted. (E) BMCs from DR4*0401 Tg and DR4*0402 Tg mice were cultured atop bone matrices in OC-differentiating conditions and matrix resorption was quantified at different time points. Black areas represent intact matrix; white areas correspond to matrix resorption. Right hand side shows quantitative data of mean ± SEM of matrix resorption. (F) BMCs from DR4*0401 Tg and DR4*0402 Tg mice were cultured atop bone slices in OC-differentiating medium. On day 10, slices were stained for TRAP (upper panel). Arrowheads point at representative OCs. Lower panel shows toluidine blue (TB)-stained bone slices after removal of attached BMCs. Arrowheads point at representative resorption pits. White horizontal bars = 100 µM. Bar graphs in the right hand side represent mean ± SEM events from quintuplicate cultures in each of the two respective panels.