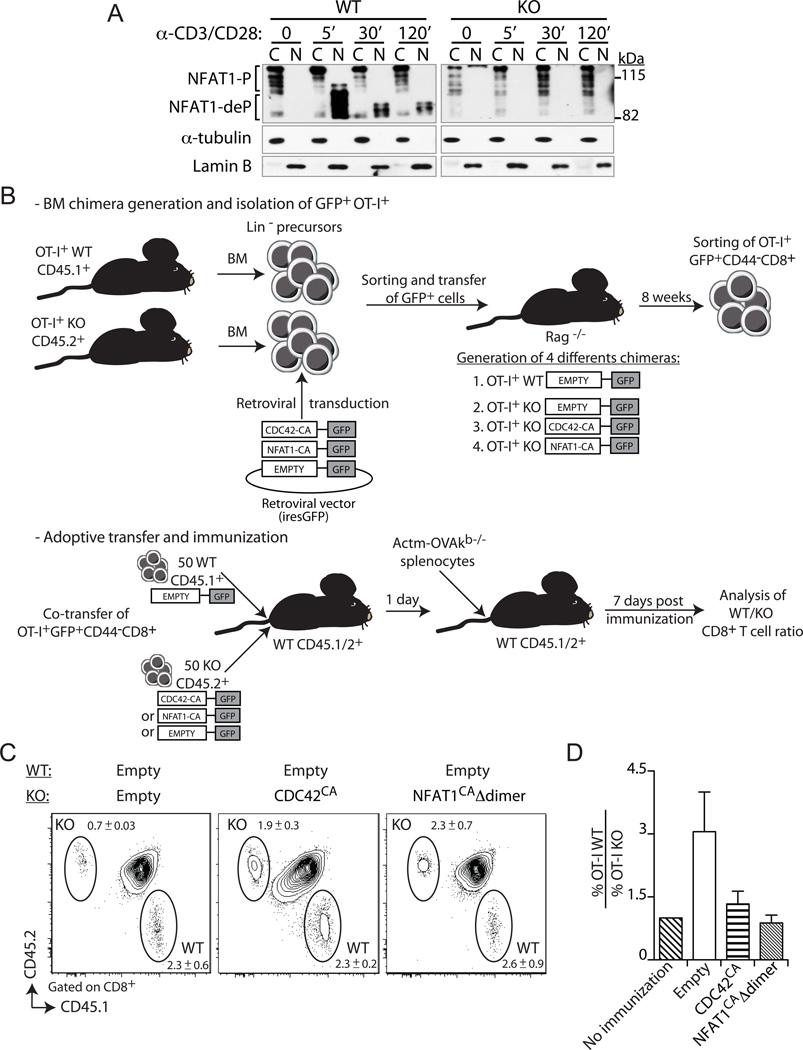
Figure 5. Constitutively active Cdc42 or NFAT1 rescue primary expansion of Def6−/− CD8+ T cells.
A, Primary WT and KO CD8+ T cells were activated with anti-CD3 plus -CD28 (5 and 2.5µg/ml, respectively) mAbs for the indicated times. Cytoplasmic (C) and nuclear (N) fractions were immunoblotted with an NFAT1-specific Ab. Fractions were also immunoblotted with α-tubulin- and lamin B-specific Abs to confirm purity of the cytosolic and nuclear fractions, respectively. NFAT1-P, phosphorylated NFAT1, NFAT1-deP, dephosphorylated NFAT1. B, Experimental setup used to assess the role of SLAT-mediated NFAT1 and CDC42 activation in CD8+ T cell expansion. The top panel describes the generation of retrogenic mice, followed by sorting of retrovirally transduced (GFP+) naïve WT or KO CD8+ T cells, which are used as donor cells for co-adoptive transfers into immunized WT recipients, as described in bottom panel. C and D, WT (CD45.1+) or KO (CD45.2+) BM progenitor cells from respective OT-I TCR-Tg mice were transduced either with the control empty retrovirus (WT and KO; Mock) or with retroviruses encoding the constitutively active Cdc42 (KO; Cdc42CA) or NFAT1 (KO; NFAT1CAΔdimer). Transduced GFP+ BM cells were sorted and injected i.v. into sublethally irradiated Rag1−/− mice. Eight weeks later, WT or KO naïve OT-I CD8+ cells (tetramer+, CD62Lhigh, and CD44low) were sorted from spleen cell suspensions. WT and KO cells (50 cells each) were transferred as indicated into recipient B6 (CD45.1/2+) mice. One day later, the recipient mice were immunized with 5 × 106 Act-mOVA/Kb−/− splenocytes and CD8+ T cell expansion of the transferred cells analyzed on day 7 post-challenge. C, Frequency of WT and KO OT-I CD8+ T cells among CD8+ T cells in the blood (mean ± SEM, n=5). D, Average ratio of WT OT-I to KO OT-I CD8+ T cells (n=5). The ratio in non-immunized animals is set at 1. Data are representative of three independents experiments.