Abstract
OBJECTIVE:
Persistent pulmonary hypertension of the newborn (PPHN) is a clinical syndrome of late-preterm and full-term infants associated with failure of the normal fetal-to-neonatal circulatory transition. This study was designed to test the hypothesis that risk for PPHN is increased after antenatal exposure to nonsteroidal antiinflammatory drugs (NSAIDs), with particular emphasis on late gestational exposures.
METHODS:
Between 1998 and 2003, we interviewed 377 women whose infants had PPHN and 836 control mothers of infants matched to cases by hospital and birth date. Interviews captured information on prescription and over-the-counter medication use in pregnancy as well as a variety of potential confounding factors. Crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for third-trimester maternal NSAID use were estimated by using multivariate conditional logistic regression.
RESULTS:
During the third trimester of gestation, 33 infants (8.8%) with PPHN were exposed to any NSAID compared with 80 (9.6%) controls (OR 0.8; 95% CI 0.5–1.3). We observed an elevated OR for PPHN risk among infants whose mothers consumed aspirin during the third-trimester; however, the lower 95% CI included the null. Neither nonaspirin NSAIDs at any time during pregnancy nor ibuprofen use during the third trimester was associated with an elevated risk of PPHN. Similarly, no association was observed between a mother’s third-trimester acetaminophen use and the occurrence of PPHN in her newborn.
CONCLUSIONS:
This large multicenter epidemiologic study of PPHN risk revealed no evidence to support the hypothesis that maternal consumption during pregnancy of NSAIDs overall or ibuprofen in particular is associated with PPHN risk.
KEY WORDS: persistent fetal circulation, pulmonary hypertension, newborn infant, epidemiology, perinatal health, nonsteroidal antiinflammatory drugs
What’s Known on This Subject:
Knowledge is limited regarding the epidemiology of persistent pulmonary hypertension of the newborn (PPHN). Previous work has implicated a host of perinatal risk factors and a few antenatal antecedents of PPHN, including maternal consumption during pregnancy of nonsteroidal antiinflammatory medications.
What This Study Adds:
In contrast to results of previous studies, we found no association between PPHN and maternal consumption during late pregnancy of nonsteroidal antiinflammatory drugs in general or ibuprofen in particular.
The pulmonary circulation of the fetus is characterized by high vascular resistance when compared with the lower pulmonary vascular resistance necessary for pulmonary gas exchange in postnatal life. As a result, the fetal circulation diverts blood that has been oxygenated by the placenta away from the fetal lungs by means of 2 channels: the ductus arteriosus (DA) and foramen ovale (FO). In the normal transition at birth from fetal-to-neonatal circulation, pulmonary vascular resistance falls, and the right-to-left DA and FO hemodynamic shunting that occurs in the normal fetus ceases. In 1 or 2 of every 1000 live births, however, there is a failure of the normal fetal-to-neonatal circulatory transition.1–3 This disruption leads to persistent pulmonary hypertension of the newborn (PPHN), a disorder characterized by postnatal persistence of elevated pulmonary vascular resistance, right-to-left shunting of blood through the fetal channels, diminished pulmonary blood flow, and profound hypoxemia.4–6 PPHN typically occurs in a full-term or late-preterm infant who has no associated congenital anomalies and becomes symptomatic within minutes or hours of birth with severe respiratory failure, is caused by vascular remodeling or a maladaptive pulmonary vascular response,3,4,7,8 and is associated with substantial morbidity and mortality.1–3,9,10
Epidemiologic associations between PPHN and perinatal factors such as meconium aspiration, pneumonia, sepsis, nonvertex presentation, and cesarean delivery are well recognized.11–13 Although some of these factors may be consequences of or, alternatively, share common antecedents with PPHN, pulmonary vascular remodeling observed among infants who had PPHN and died soon after birth14 suggests that antenatal exposures might contribute to the development of PPHN.
The hypothesis that antenatal maternal consumption of nonsteroidal antiinflammatory drug (NSAIDs) could lead to PPHN is based on the demonstration, in both animals9,15,16 and humans,17,18 of fetal or neonatal DA constriction with salicylate9,15 or indomethacin.16–18 Human case reports have linked the occurrence of PPHN with maternal NSAID treatment.17,19–26 In 1996, a small epidemiologic study found an increase in PPHN risk (odds ratio [OR] = 6.2, 95% confidence interval [CI] 1.8–21.8) associated with antenatal exposure to NSAIDs, a finding that was based on just 7 exposed cases, only 4 of whom reported third-trimester use.11 In 2001, another study reported a significant association between PPHN and the presence of NSAIDs (ie, aspirin, ibuprofen, indomethacin, naproxen) in meconium (OR 21.5, calculated from table 4 in Alano et al).27
Methods
Study Design
To test the hypothesis that late pregnancy exposure to NSAIDs is associated with an increased risk of PPHN, we conducted a case-control study of risk factors for PPHN among infants identified through the Slone Epidemiology Center’s Birth Defects Study (BDS), an ongoing surveillance program of risk factors for birth defects.28 The PPHN study component was designed specifically to rigorously identify infants with PPHN and evaluate risk factors for PPHN, with particular emphasis on antenatal NSAID exposure.
Study Population
Study subjects were drawn from 97 institutions in 4 metropolitan areas (Boston, Philadelphia, San Diego, and Toronto) between 1998 and 2003. They were identified through review of admissions and discharges at major referral hospitals and clinics, logbooks in NICUs and through weekly telephone contact with collaborators at newborn nurseries in community hospitals (the latter to identify infants with PPHN who might not have been referred to major centers). Healthy newborns from the same centers also were enrolled. Institutional review board approval was obtained from all participating institutions. All mothers who were interviewed provided consent.
Designation of Case Status
All infants admitted to the newborn intensive care units (NICUs) at participating hospitals were screened by BDS nurses or respiratory therapists trained to identify infants with respiratory signs or diagnoses that made them candidates for having PPHN. To determine eligibility and identify infants who met criteria for PPHN, 1 investigator (LVM) who was masked to maternal exposure history, reviewed the medical records of all infants with diagnostic codes for asphyxia, cyanotic congenital heart disease, respiratory distress syndrome, pneumonia, meconium aspiration, transient tachypnea of the newborn, persistent fetal circulation, or pulmonary hypertension. Because we excluded infants with major congenital malformations from our analyses, those with known anomalies such as congenital heart disease or pulmonary hypoplasia were ineligible.
PPHN cases were defined as follows:
Gestational age >34 weeks. Gestational age, as reported in the infant’s medical record at the time of ascertainment, was verified by comparing the date of birth to the due date reported by the mother, which was based on her last menstrual period or early pregnancy ultrasound estimate.
Presentation shortly after birth with severe respiratory failure, defined as the need for intubation and mechanical ventilation.
Evidence of pulmonary hypertension as documented either by a ≥5% gradient between preductal and postductal oxygen saturation or by echocardiographic evidence. Among those who underwent echocardiography, infants were designated as having PPHN if the cardiologist evaluating the echocardiogram assigned the diagnosis of PPHN or noted marked pulmonary hypertension or if the echocardiogram showed right-to-left hemodynamic shunting at the FO or DA or bidirectional hemodynamic shunting accompanied by leftward bowing of the ventricular septum to a degree consistent with pulmonary arterial pressure that was greater than half of the systemic pressure.
A potential case subject was excluded from eligibility for a PPHN diagnosis if he or she had evidence of any congenital cardiothoracic abnormality except for patent ductus arteriosus, patent foramen ovale, atrial septal defect, or a single, small, muscular ventriculoseptal defect.
The ability to acquire the quality and quantity of data needed to determine case status varied among medical centers. In response, potential PPHN cases were subsequently classified as
“confirmed” if they met every criterion for the case definition of PPHN;
“probable” if their clinical courses were highly suggestive of PPHN and their diagnostic criteria for PPHN approximated, but did not fall within, the specified ranges;
“possible” if their clinical courses were consistent with PPHN but appropriate diagnostic tests were never performed or were unavailable; or
“not a case” if their clinical or diagnostic tests were inconsistent with PPHN.
Subjects were excluded from consideration if they lacked information judged necessary to classify the subject into one of the categories above. Only confirmed and probable cases were included in the main analyses.
Selection of Controls
The control group included infants born after 34 weeks’ gestation without a congenital abnormality or a respiratory problem who were matched to cases by birth hospital and calendar date of birth (±30 days). We initially selected up to 4 potential controls per case and interviewed on average 2.2 controls per case. After final classification of PPHN cases and completion of interviews, controls who were matched to confirmed and probable cases and those who had completed interviews were selected for the analyses.
Assessment of Exposure
Within 6 months of delivery, trained study nurses who were unaware of the hypothesis interviewed the mothers of the case and control infants. The telephone interview was detailed and structured and included questions on demographic characteristics, the mother’s medical and obstetrical history, parents’ habits and occupations, and a history of the use of all medications (prescription and over the counter [OTC]) from the period 2 months before conception throughout the entire pregnancy. The interview was computer-based, with direct entry of responses and access to dictionaries (ie, drugs and diagnoses) and instantaneous coding. Recall of specific analgesic/antipyretic products was aided by the BDS Medication Identification Booklet containing pictures of available OTC products.
Drug use and illness information were collected by using a 4-level approach for obtaining medication information (by illness, medication category, symptom, and drug prompts) that has been previously described.29,30 This method ensures more complete reporting29,31 and allows linkage between all medications and occurrences of illnesses.
We defined late pregnancy exposure as use of NSAIDs anytime in the third trimester. We classified NSAIDs as either aspirin or nonaspirin NSAIDs and considered specific nonaspirin NSAIDs (mainly ibuprofen). Because the third trimester is shorter among infants born preterm, thereby reducing the opportunity for exposure during this trimester, we also considered the use of NSAIDs during the month preceding the delivery (time-varying exposure), and repeated the analysis among infants born at full-term gestation (≥37 weeks postmenstrual age). Because 1 epidemiologic study found the increased PPHN risk after first trimester exposure,11 we also evaluated the effect of NSAID use for each trimester. Finally, because low-dose aspirin is used to treat some pregnancy-related conditions and NSAID treatment is used to arrest preterm labor, we evaluated the potential effect of these indications for treatment.
Data Analysis
In this analysis, we sought to evaluate 2 principal study hypotheses: (1) maternal consumption during pregnancy of NSAIDs, but not acetaminophen, is associated with an increased risk of the infant developing PPHN and (2) the association between NSAIDs and increased PPHN risk is most evident when the NSAID consumption occurs during the third trimester.
Matched ORs and their 95% CIs were estimated for PPHN by using multivariate conditional logistic regression with separate terms for the 3 medication exposures, race/ethnicity (ie, black, Hispanic, Asian, others, and white as referent), diabetes mellitus, prepregnancy BMI (ie, 20–27, >27, unknown, and <20 as referent), hypertension, and multiple birth. Analyses were performed by using SAS for Windows, version V.8.2.
Using the same data set, we previously tested the hypothesis that selective serotonin reuptake inhibitors increased the risk of PPHN.30
Results
Of 843 term or near-term newborn infants we identified with diagnoses of asphyxia, cyanotic congenital heart disease, respiratory distress syndrome, pneumonia, meconium aspiration, transient tachypnea of the newborn, persistent fetal circulation, or pulmonary hypertension, 377 were classified as having confirmed or probable PPHN. They were matched to 836 controls (Table 1). The participation rate was 69% for mothers of PPHN subjects and 68% for mothers of controls. After exclusion of mothers who could not be located and invited to participate, the rates were 73% and 71%, respectively.
TABLE 1.
Categorization of the Study Population: Control Subjects and Subjects Referred as Potential PPHN Cases, 58% of Whom Were Designated Confirmed or Probable PPHN Casesa
| Case or Control Status | Frequency | Percent of Eligible Study Population |
|---|---|---|
| Controls | 9032 | 85.99 |
| Matched control subjects | 836 | 7.91 |
| PPHN cases | 377 | |
| Confirmed PPHN | 337 | 3.20 |
| Probable case | 40 | 0.36 |
| Potential PPHN cases | 268 | |
| Ascertained PPHN | 5 | 0.10 |
| Possible case | 28 | 0.27 |
| Indeterminate case | 37 | 0.36 |
| Not a case | 198 | 1.81 |
The final study population consisted of 377 PPHN cases and 836 matched controls.
Demographic, prenatal, and perinatal factors associated with PPHN in this study population have been previously described.29 Those that were considered potential confounding factors in analyses of the relationships among analgesics and PPHN are presented in Table 2. Factors associated with increased risk of PPHN included maternal race/ethnicity (ie, Black or Asian), BMI >27, maternal diabetes mellitus or asthma, cesarean delivery, male infant gender, birth weight <2500 or >4000 g, gestational age <37 or >41 weeks, and large for gestational age designation. Reduced PPHN risk was observed for infants whose mothers had received >15 years of education.
TABLE 2.
Demographic, Pregnancy, and Neonatal Factors and Their Relationship With PPHN
| Variable | PPHN N (%) | Matched Controls N (%) | Crude Matched OR (95% CI) | Adjusted Ia OR (95% CI) | Adjusted IIb OR (95% CI) |
|---|---|---|---|---|---|
| Demographic factors | |||||
| Maternal race | |||||
| White | 217 (57.6) | 606 (72.5) | Reference | Reference | Reference |
| Black | 70 (18.6) | 75 (9.0) | 3.0 (1.9–4.7) | 2.5 (1.5–4.1) | 2.5 (1.4–4.5) |
| Asian | 32 (8.5) | 42 (5.0) | 2.3 (1.4–3.8) | 2.5 (1.4–4.4) | 2.9 (1.5–5.7) |
| Hispanic | 45 (12.0) | 91 (11.0) | 1.4 (0.9–2.1) | 1.2 (0.8–2.0) | 1.1 (0.6–1.9) |
| Other | 13 (3.5) | 22 (2.6) | 2.0 (1.0–4.1) | 1.7 (0.8–3.7) | 2.7 (1.0–7.0) |
| Maternal age | |||||
| ≤25 | 99 (26.3) | 208 (24.9) | Reference | Reference | Reference |
| 25–30 | 104 (27.6) | 241 (28.8) | 1.0 (0.7–1.4) | 1.3 (0.9–1.9) | 1.0 (0.6–1.7) |
| 30–35 | 112 (29.7) | 261 (31.2) | 1.0 (0.7–1.4) | 1.2 (0.8–1.9) | 1.0 (0.6–1.6) |
| >35 | 62 (16.5) | 126 (15.1) | 1.1 (0.8–1.7) | 1.5 (0.9–2.4) | 1.2 (0.7–2.0) |
| Maternal education | |||||
| <13 | 135 (35.8) | 228 (27.3) | Reference | Reference | Reference |
| 13–15 | 106 (28.1) | 234 (28.0) | 0.8 (0.6–1.1) | 0.8 (0.5–1.1) | 0.9 (0.6–1.4) |
| >15 | 136 (36.1) | 374 (44.7) | 0.6 (0.5–0.9) | 0.7 (0.5–1.0) | 0.8 (0.5–1.3) |
| Parity | |||||
| Primiparous | 250 (66.3) | 561 (67.1) | Reference | Reference | Reference |
| Multiparous | 127 (33.7) | 275 (32.9) | 1.0 (0.8–1.3) | 1.2 (0.9–1.6) | 1.0 (0.7–1.4) |
| BMI | |||||
| <20 | 38 (10.1) | 152 (18.2) | Reference | Reference | Reference |
| 20–27 | 197 (52.3) | 497 (59.5) | 1.5 (1.0–2.3) | 1.5 (1.0–2.3) | 1.3 (0.8–2.0) |
| >27 | 134 (35.5) | 174 (20.8) | 3.0 (1.9–4.6) | 2.5 (1.6–4.0) | 1.7 (1.0–2.9) |
| Unknown | 8 (2.1) | 13 (1.6) | 2.2 (0.8–5.9) | 2.0 (0.7–5.5) | 0.9 (0.2–3.2) |
| Pregnancy factors | |||||
| Maternal smoking | |||||
| Never | 226 (60.0) | 494 (59.1) | Reference | Reference | Reference |
| Before pregnancy | 84 (22.3) | 206 (24.6) | 0.9 (0.7–1.2) | 0.9 (0.6–1.3) | 0.9 (0.6–1.4) |
| During pregnancy | 67 (17.8) | 136 (16.3) | 1.1 (0.8–1.5) | 1.1 (0.7–1.6) | 1.1 (0.7–1.7) |
| Multiple gestation | |||||
| Singleton | 365 (96.8) | 819 (98.0) | Reference | Reference | Reference |
| Multiple | 12 (3.2) | 17 (2.0) | 1.6 (0.8–3.5) | 1.3 (0.6–2.9) | 0.4 (0.1–1.0) |
| Infant gender | |||||
| Female | 138 (36.6) | 426 (51.0) | Reference | Reference | Reference |
| Male | 239 (63.4) | 410 (49.0) | 1.9 (1.4–2.4) | 1.6 (1.2–2.2) | 1.5 (1.1–2.1) |
| Asthma | |||||
| Yes vs No | 46 (12.2) | 62 (7.4) | 1.7 (1.2–2.6) | 1.6 (1.0–2.5) | 2.0 (1.2–3.4) |
| Hypertension | |||||
| Yes vs No | 66 (17.5) | 90 (10.8) | 1.8 (1.3–2.6) | 1.3 (0.9–1.9) | 1.3 (0.8–2.1) |
| Diabetes mellitus | |||||
| Yes vs No | 37 (9.8) | 35 (4.2) | 2.4 (1.5–3.8) | 1.6 (0.9–2.8) | 1.2 (0.6–2.3) |
| NSAIDs third trimester | |||||
| Yes vs No | 37 (9.8) | 87 (10.4) | 1.0 (0.6–1.5) | 0.8 (0.5–1.3) | 0.6 (0.4–1.1) |
| Perinatal factors | |||||
| Delivery | |||||
| Vaginal | 146 (38.7) | 677 (81.0) | Reference | Reference | |
| Cesarean delivery | 231 (61.3) | 159 (19.0) | 7.5 (5.5–10.4) | 7.0 (4.9–10.1) | |
| Gestational age (wk) | |||||
| <37 | 60 (15.9) | 42 (5.0) | 3.8 (2.5–5.8) | 3.6 (2.0–6.5) | |
| 37-41 | 227 (60.2) | 670 (80.1) | Reference | Reference | |
| >41 | 90 (23.9) | 124 (14.8) | 2.2 (1.6–3.0) | 2.1 (1.4–3.1) | |
| Birth wt (g) | |||||
| <2500 | 29 (7.7) | 29 (3.5) | 2.4 (1.4–4.1) | 1.1 (0.5–2.4) | |
| 2500–4000 | 275 (72.9) | 704 (84.3) | Reference | Reference | |
| >4000 | 73 (19.4) | 102 (12.2) | 2.0 (1.4–2.8) | 1.7 (1.1–2.6) |
Adjusted for other covariates within demographic and pregnancy factors.
Adjusted for all the covariates in the table.
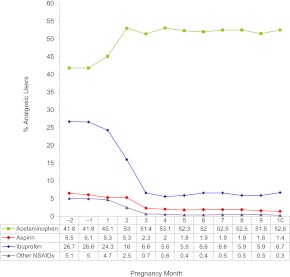
Among matched control subjects, overall rates of exposure at any time during pregnancy were 71.7% for acetaminophen, 28.3% for ibuprofen, 6.9% for aspirin, and 5.4% for other NSAIDs. Exposure to analgesics varied during pregnancy, and the observed patterns differed both by medication and pregnancy trimester (Fig 1). In the first trimester, acetaminophen use increased and the use of other analgesics dropped considerably. At the end of the first trimester, acetaminophen use was 51% and ibuprofen, aspirin, and intake of other NSAIDs had fallen to 6%, 2.3%, and 0.7%, respectively. Acetaminophen and ibuprofen exposure remained relatively constant throughout the remainder of pregnancy. In the third trimester, however, aspirin and other NSAID use fell to 1.7% and 0.45%, respectively. Although third-trimester acetaminophen use was somewhat lower for those in the Toronto and San Diego centers than in the Boston or Philadelphia centers, the third trimester patterns of consumption of aspirin, ibuprofen, and other NSAIDs were relatively consistent across all 4 centers (data not shown).
FIGURE 1.
Trends in analgesic use during pregnancy. These data reflect the use of selected analgesics among matched control subjects by pregnancy month. The denominator at each time point reflects women pregnant at that gestational month.
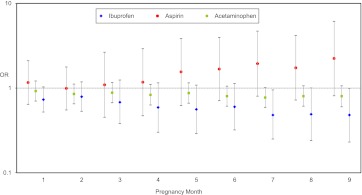
We first considered all infants irrespective of gestational age at birth. Figure 2 shows ORs and 95% CIs for conditional logistic regression, by pregnancy month of exposure. Table 3 presents the results of conditional logistic regression analyses for matched data associated with third-trimester exposure. The crude third-trimester estimates were 1.82 for aspirin, 0.65 for ibuprofen, and 0.8 for acetaminophen; all the 95% CI bounds included the null. Additional analyses involved a series of sequential models that adjusted for potential confounders, late preterm birth (gestational age 34–37 weeks), and cesarean delivery. Although prematurity and cesarean delivery cannot be true confounders because they occur after prenatal NSAID exposure and possibly after the pathologic features of the outcome, we explored whether they accounted for any of the observed associations as intermediate variables or were proxies for unmeasured confounders. ORs remained largely unchanged for ibuprofen and acetaminophen but decreased for aspirin to 1.19 (0.50–2.87). When the analyses were restricted to infants born at or beyond 37 weeks of gestation (Table 4), the adjusted OR for ibuprofen was 0.42 (0.20–0.89), for aspirin was 1.47 (0.52–4.12), and for acetaminophen was 0.90 (0.64–1.27). Analysis of time-varying exposures that considered drug use during the gestational month preceding delivery rather than during the third trimester did not yield meaningfully different results (data not shown).
FIGURE 2.
Association between PPHN and antiinflammatory drug use by pregnancy month. Conditional logistic regression for matched data showing ORs and 95% CIs adjusted for maternal race, diabetes, pre-pregnancy BMI, and gestational hypertension.
TABLE 3.
Association Between PPHN and Selected Analgesics Used During the Third Trimester
| PPHN, N (%) | Controls, N (%) | Crude OR (95% CI) | Adjusted ORa (95% CI) | Adjusted ORb (95% CI) | Adjusted ORc (95% CI) | |
|---|---|---|---|---|---|---|
| Ibuprofen | 18 (4.8) | 64 (7.7) | 0.65 (0.38–1.12) | 0.56 (0.31–1.02) | 0.58 (0.32–1.05) | 0.56 (0.28–1.11) |
| Aspirin | 15 (4.0) | 19 (2.3) | 1.82 (0.92–3.62) | 1.69 (0.77–3.69) | 1.66 (0.74–3.74) | 1.19 (0.50–2.87) |
| Acetaminophen | 191 (50.7) | 475 (56.9) | 0.80 (0.63–1.03) | 0.86 (0.66–1.12) | 0.87 (0.66–1.14) | 0.86 (0.63–1.17) |
Conditional logistic regression for matched data.
Adjusted for race, diabetes, pre-pregnancy BMI, hypertension, and multiple births.
Adjusted for preterm birth, in addition to variables noted in a.
Adjusted for cesarean delivery, in addition to variables noted in a and b.
TABLE 4.
Association Between PPHN and Selected Analgesics Used During the Third Trimester
| PPHN, N (%) | Controls, N (%) | Crude OR (95% CI) | Adjusted ORa (95% CI) | Adjusted ORb (95% CI) | |
|---|---|---|---|---|---|
| Ibuprofen | 15 (4.7) | 62 (7.8) | 0.55 (0.30–0.99) | 0.40 (0.21–0.78) | 0.42 (0.20–0.89) |
| Aspirin | 12 (3.8) | 17 (2.1) | 2.01 (0.91–4.46) | 2.34 (0.94–5.88) | 1.47 (0.52–4.12) |
| Acetaminophen | 159 (50.2) | 456 (57.3) | 0.83 (0.63–1.09) | 0.86 (0.64–1.16) | 0.90 (0.64–1.27) |
Conditional logistic regression for matched data. Restricted to term births (≥37 wk).
Adjusted for race, diabetes, pre-pregnancy BMI, hypertension, and multiple births.
Adjusted for cesarean delivery, in addition to variables noted in a.
Discussion
Despite biological plausibility, supportive laboratory work, and some epidemiologic evidence, in the current multicenter study, we found no consistent support for the hypothesis that a mother’s NSAID consumption during pregnancy increases the risk of her infant developing PPHN. Although the risk associated with aspirin exposure increased as gestation progressed (maximum OR 2.34; 95% CI 0.94–5.88), the lower confidence bound did not exclude the null. Unexpectedly, among infants born at or beyond 37 weeks’ gestation, maternal ibuprofen consumption in the third trimester of pregnancy was associated with an ∼50% reduced risk of PPHN (OR 0.42; 95% CI 0.20–0.89).
Our negative findings for NSAIDs as a risk factor for PPHN are puzzling given the biological plausibility for such an association. NSAIDs inhibit prostaglandin synthesis, inducing fetal ductus arteriosus constriction,16–18,21 increasing pulmonary blood flow, and remodeling the pulmonary vasculature,9,32,33 resulting in postnatal pulmonary hypertension.34 Furthermore, the pattern of pulmonary vascular morphologic changes described in animals exposed in utero to prostaglandin inhibitors9,32 is similar to that found in a subset of infants with PPHN.14 On the other hand, in utero constriction of the DA with prostaglandin inhibitors also appears to produce local hypoxia and remodeling of the vessel, changes that decrease ductal responsiveness and contractility after birth35 resulting in patent ductus arteriosus, would not lead to PPHN, however.
Existing epidemiologic evidence linking antenatal NSAID exposure and PPHN is limited and based on smaller studies lacking the power and methodologic rigor of the current study. The link between maternal NSAID consumption during pregnancy and PPHN is based largely on a series of case reports. The only previous epidemiologic study showing a positive association between antenatal NSAID exposure and PPHN11 was based on only 26 infants whose mothers reported NSAID intake at any time during pregnancy and just 4 mothers (1 case and 3 controls; 1% for each) who reported taking NSAIDs during the last trimester of pregnancy. That exploratory study found elevated ORs (OR 3.6, 95% CI 1.2–11 among all subjects and OR 8.1, 95% CI 2.3–28 among the inborn subjects) despite relying on maternal recall, finding unexpectedly low prevalence of NSAID use, and including early pregnancy exposures of questionable biological relevance. At the other extreme, studies based on serum36 or meconium27 salicylate levels found a surprisingly high prevalence of exposure (50%) and an extremely low concordance between biological samples and maternal recall (only 2 of 6 positive mothers in one study36 and 1 of 44 in another27 reported ingestion of aspirin). The authors of these studies attributed their findings to maternal failure to recall or recognize salicylates in multiingredient OTC medications.27 Of note, in the meconium-based study, 33% of the “randomly selected healthy controls” were born by cesarean delivery, and 59% had meconium-stained amniotic fluid, which raises the possibility of biased control selection. Moreover, this study did not replicate the previously established associations between PPHN and postmaturity, cesarean delivery, meconium staining of the amniotic fluid, male gender of the infant, or African American ethnicity.10–13,37,38 One recent study of the association between selective serotonin reuptake inhibitors and PPHN risk from 5 Nordic countries observed no association between NSAID use and PPHN.39
The main limitation of the current study is the reliance on maternal recall for information about exposure status. Several factors suggest, however, that recall bias is an unlikely explanation for our study results. First, if medication use in general was more likely to be recalled by mothers of cases than controls, an elevated risk associated with acetaminophen would be expected; this was not observed. Furthermore, there was no evidence of public perception of increased risk of PPHN associated with maternal intake of aspirin or reduced PPHN risk associated with ibuprofen consumption. We recognize that there is no gold standard for identifying exposure to OTC medications in pregnancy and carefully conducted maternal interviews are considered a reasonable way to capture such exposures. Nonetheless, misclassification is a potential source of bias, particularly for the medications under study. It is unlikely, however, that random misclassification (ie, unrelated to the outcome) would explain the differences observed between aspirin and nonaspirin (ie, acetaminophen, ibuprofen) products.
The failure of the current study to detect an association between maternal NSAID intake during pregnancy and neonatal PPHN runs counter to evidence in laboratory animals and human case reports. However, differential effects of various specific NSAIDs (eg, increased PPHN risk associated with aspirin but not other NSAIDs) is biologically plausible. The apparent reduction in PPHN risk with antenatal exposure to ibuprofen is particularly intriguing, however, and is not explained by the drug’s propensity to cause DA closure. The role of inflammation in the pathogenesis of pulmonary hypertension is receiving increasing recognition,40–44 and we speculate that the protective effect of antenatal exposure to ibuprofen in PPHN, if real, is more likely to be attributable to its antiinflammatory properties than to a direct effect on the pulmonary vasculature. If antenatal ibuprofen exposure mitigates perinatal inflammation, it might reduce the occurrence of PPHN.
Conclusions
The current study represents the largest epidemiologic study of risk factors for PPHN and was designed specifically to evaluate the hypothesis that antenatal NSAID exposure is associated with an increased risk of an infant developing PPHN. Strengths of the study are its large and geographically diverse population, thorough review and diagnostic categorization of PPHN cases, and structured interviews conducted within 6 months of each baby’s birth by highly experienced interviewers using rigorous methods for medication identification.
In this large case-control study of PPHN, we found no support for the previous hypothesis that antenatal exposure to NSAIDs increases the risk of PPHN. Multivariable analyses of third-trimester exposures, adjusting for other risk factors, revealed an increase in risk of the occurrence of PPHN associated with antenatal exposure to aspirin intake, with confidence limits that did not exclude the null and an unexpected reduction in PPHN occurrence associated with third-trimester maternal intake of ibuprofen.
Acknowledgments
The authors thank Fiona Rice, MPH, Dawn Jacobs, RN, MPH, Rachel Wilson, MPH, Sally Perkins, RN, Kathleen Sheehan, RN, Karen Bennett Mark, RN, Deborah Kasindorf, RN, Clare Coughlin, RN, Geraldine Ellison, RN, Joan Shander, Diane Gallagher, Nastia Dynkin, Nancy Rodriquez-Sheridan, Cecilia Stadler, Meghan Malone-Moses, Melody Kisor, Dawn Taggett, MPH, Sherlonda Allen, Michelle Hose, RN, Beth Smith, RN, Patricia Maloney, RN, Merianne Mitchell, RT, Valerie Hillis, and the late Rita Krolak, RN, for their assistance in data collection and computer programming, as well as Lindsay Phillips Hage for manuscript preparation. Thanks also to Drs Christina Chambers and Ken Lyons Jones for leading the San Diego center. Finally, the authors extend their deepest appreciation to the mothers who participated in this study and the medical and nursing staff at each participating hospital who encouraged and supported our research efforts.
Glossary
- BDS
Birth Defects Study
- CI
confidence interval
- DA
ductus arteriosus
- FO
foramen ovale
- NSAID
nonsteroidal antiinflammatory drug
- OR
odds ratio
- OTC
over the counter
- PPHN
persistent pulmonary hypertension of the newborn
Footnotes
All authors made substantive intellectual contributions to this study; each has contributed sufficiently in the work to take public responsibility for appropriate portions of the content; and each has seen and approved the final submission version of the manuscript.
FINANCIAL DISCLOSURE: Dr Werler provided consulting to Abbott Laboratories on their study of adalmiumab use among pregnant women and pregnancy outcomes. Abbott Laboratories also makes products that contain ibuprofen. Dr Hernandez-Diaz has consulted as an advisor for pregnancy registries sponsored by Novartis and GlaxoSmithKline unrelated to the research presented in the article. However, these companies manufacture antiinflammatory medications. Dr Mitchell has a financial interest in Johnson & Johnson valued at less than $20,000. Johnson & Johnson makes both acetaminophen and ibuprofen products; however, the company provided no support for this project and had no role in any aspect of the research or manuscript preparation. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by National Institutes of Health grant HL58763. Funded by the National Institutes of Health (NIH).
References
- 1.Farrow KN, Fliman P, Steinhorn RH. The diseases treated with ECMO: focus on PPHN. Semin Perinatol. 2005;29(1):8–14 [DOI] [PubMed] [Google Scholar]
- 2.Hageman JR, Adams MA, Gardner TH. Persistent pulmonary hypertension of the newborn. Trends in incidence, diagnosis, and management. Am J Dis Child. 1984;138(6):592–595 [DOI] [PubMed] [Google Scholar]
- 3.Walsh-Sukys MC, Tyson JE, Wright LL, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105(1 pt 1):14–20 [DOI] [PubMed] [Google Scholar]
- 4.Abman SH. New developments in the pathogenesis and treatment of neonatal pulmonary hypertension. Pediatr Pulmonol Suppl. 1999;18:201–204 [PubMed] [Google Scholar]
- 5.Gersony WM, Duc GV, Sinclair JC. “PFC” syndrome: Persistence of the fetal circulation. Circulation. 1969;40:111 [Google Scholar]
- 6.Walsh-Sukys MC. Persistent pulmonary hypertension of the newborn. The black box revisited. Clin Perinatol. 1993;20(1):127–143 [PubMed] [Google Scholar]
- 7.Clark RH, Huckaby JL, Kueser TJ, et al. Clinical Inhaled Nitric Oxide Research Group . Low-dose nitric oxide therapy for persistent pulmonary hypertension: 1-year follow-up. J Perinatol. 2003;23(4):300–303 [DOI] [PubMed] [Google Scholar]
- 8.Glass P, Wagner AE, Papero PH, et al. Neurodevelopmental status at age five years of neonates treated with extracorporeal membrane oxygenation. J Pediatr. 1995;127(3):447–457 [DOI] [PubMed] [Google Scholar]
- 9.Levin DL, Mills LJ, Weinberg AG. Hemodynamic, pulmonary vascular, and myocardial abnormalities secondary to pharmacologic constriction of the fetal ductus arteriosus. A possible mechanism for persistent pulmonary hypertension and transient tricuspid insufficiency in the newborn infant. Circulation. 1979;60(2):360–364 [DOI] [PubMed] [Google Scholar]
- 10.Dakshinamurti S. Pathophysiologic mechanisms of persistent pulmonary hypertension of the newborn. Pediatr Pulmonol. 2005;39(6):492–503 [DOI] [PubMed] [Google Scholar]
- 11.Van Marter LJ, Leviton A, Allred EN, et al. Persistent pulmonary hypertension of the newborn and smoking and aspirin and nonsteroidal antiinflammatory drug consumption during pregnancy. Pediatrics. 1996;97(5):658–663 [PubMed] [Google Scholar]
- 12.Reece EA, Moya F, Yazigi R, Holford T, Duncan C, Ehrenkranz RA. Persistent pulmonary hypertension: assessment of perinatal risk factors. Obstet Gynecol. 1987;70(5):696–700 [PubMed] [Google Scholar]
- 13.Bearer C, Emerson RK, O’Riordan MA, Roitman E, Shackleton C. Maternal tobacco smoke exposure and persistent pulmonary hypertension of the newborn. Environ Health Perspect. 1997;105(2):202–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy JD, Rabinovitch M, Goldstein JD, Reid LM. The structural basis of persistent pulmonary hypertension of the newborn infant. J Pediatr. 1981;98(6):962–967 [DOI] [PubMed] [Google Scholar]
- 15.Heymann MA, Rudolph AM, Silverman NH. Closure of the ductus arteriosus in premature infants by inhibition of prostaglandin synthesis. N Engl J Med. 1976;295(10):530–533 [DOI] [PubMed] [Google Scholar]
- 16.Levin DL, Mills LJ, Parkey M, Garriott J, Campbell W. Constriction of the fetal ductus arteriosus after administration of indomethacin to the pregnant ewe. J Pediatr. 1979;94(4):647–650 [DOI] [PubMed] [Google Scholar]
- 17.Moise KJ, Jr, Huhta JC, Sharif DS, et al. Indomethacin in the treatment of premature labor. Effects on the fetal ductus arteriosus. N Engl J Med. 1988;319(6):327–331 [DOI] [PubMed] [Google Scholar]
- 18.Eronen M, Pesonen E, Kurki T, Ylikorkala O, Hallman M. The effects of indomethacin and a beta-sympathomimetic agent on the fetal ductus arteriosus during treatment of premature labor: a randomized double-blind study. Am J Obstet Gynecol. 1991;164(1 pt 1):141–146 [DOI] [PubMed] [Google Scholar]
- 19.Manchester D, Margolis HS, Sheldon RE. Possible association between maternal indomethacin therapy and primary pulmonary hypertension of the newborn. Am J Obstet Gynecol. 1976;126(4):467–469 [DOI] [PubMed] [Google Scholar]
- 20.Csaba IF, Sulyok E, Ertl T. Relationship of maternal treatment with indomethacin to persistence of fetal circulation syndrome. J Pediatr. 1978;92(3):484. [DOI] [PubMed] [Google Scholar]
- 21.Goudie BM, Dossetor JF. Effect on the fetus of indomethacin given to suppress labour. Lancet. 1979;2(8153):1187–1188 [DOI] [PubMed] [Google Scholar]
- 22.Rubaltelli FF, Chiozza ML, Zanardo V, Cantarutti F. Effect on neonate of maternal treatment with indomethacin. J Pediatr. 1979;94(1):161. [DOI] [PubMed] [Google Scholar]
- 23.Besinger RE, Niebyl JR, Keyes WG, Johnson TR. Randomized comparative trial of indomethacin and ritodrine for the long-term treatment of preterm labor. Am J Obstet Gynecol. 1991;164(4):981–986, discussion 986–988 [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson AR, Aynsley-Green A, Mitchell MD. Persistent pulmonary hypertension and abnormal prostaglandin E levels in preterm infants after maternal treatment with naproxen. Arch Dis Child. 1979;54(12):942–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner GR, Levin DL. Prostaglandin synthesis inhibition in persistent pulmonary hypertension of the newborn. Clin Perinatol. 1984;11(3):581–589 [PubMed] [Google Scholar]
- 26.Norton ME, Merrill J, Cooper BA, Kuller JA, Clyman RI. Neonatal complications after the administration of indomethacin for preterm labor. N Engl J Med. 1993;329(22):1602–1607 [DOI] [PubMed] [Google Scholar]
- 27.Alano MA, Ngougmna E, Ostrea EM, Jr, Konduri GG. Analysis of nonsteroidal antiinflammatory drugs in meconium and its relation to persistent pulmonary hypertension of the newborn. Pediatrics. 2001;107(3):519–523 [DOI] [PubMed] [Google Scholar]
- 28.Mitchell AA, Rosenberg L, Shapiro S, Slone D. Birth defects related to bendectin use in pregnancy. I. Oral clefts and cardiac defects. JAMA. 1981;245(22):2311–2314 [PubMed] [Google Scholar]
- 29.Hernández-Díaz S, Van Marter LJ, Werler MM, Louik C, Mitchell AA. Risk factors for persistent pulmonary hypertension of the newborn. Pediatrics. 2007;120(2). Available at: www.pediatrics.org/cgi/content/full/113/2/e87/120/2/e272 [DOI] [PubMed] [Google Scholar]
- 30.Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–587 [DOI] [PubMed] [Google Scholar]
- 31.Mitchell AA, Cottler LB, Shapiro S. Effect of questionnaire design on recall of drug exposure in pregnancy. Am J Epidemiol. 1986;123(4):670–676 [DOI] [PubMed] [Google Scholar]
- 32.Harker LC, Kirkpatrick SE, Friedman WF, Bloor CM. Effects of indomethacin on fetal rat lungs: a possible cause of persistent fetal circulation (PFC). Pediatr Res. 1981;15(2):147–151 [DOI] [PubMed] [Google Scholar]
- 33.Levin DL, Fixler DE, Morriss FC, Tyson J. Morphologic analysis of the pulmonary vascular bed in infants exposed in utero to prostaglandin synthetase inhibitors. J Pediatr. 1978;92(3):478–483 [DOI] [PubMed] [Google Scholar]
- 34.Morin FC, III. Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res. 1989;25(3):245–250 [DOI] [PubMed] [Google Scholar]
- 35.Clyman RI, Chen YQ, Chemtob S, et al. In utero remodeling of the fetal lamb ductus arteriosus: the role of antenatal indomethacin and avascular zone thickness on vasa vasorum proliferation, neointima formation, and cell death. Circulation. 2001;103(13):1806–1812 [DOI] [PubMed] [Google Scholar]
- 36.Perkin RM, Levin DL, Clark R. Serum salicylate levels and right-to-left ductus shunts in newborn infants with persistent pulmonary hypertension. J Pediatr. 1980;96(4):721–726 [DOI] [PubMed] [Google Scholar]
- 37.Levine EM, Ghai V, Barton JJ, Strom CM. Mode of delivery and risk of respiratory diseases in newborns. Obstet Gynecol. 2001;97(3):439–442 [DOI] [PubMed] [Google Scholar]
- 38.Jaillard S, Houfflin-Debarge V, Storme L. Higher risk of persistent pulmonary hypertension of the newborn after cesarean. J Perinat Med. 2003;31(6):538–539 [DOI] [PubMed] [Google Scholar]
- 39.Kieler H, Artama M, Engeland A, et al. Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countries. BMJ. 2012;344:d8012. [DOI] [PubMed] [Google Scholar]
- 40.Soukka H, Rautanen M, Halkola L, Kero P, Kääpä P. Meconium aspiration induces ARDS-like pulmonary response in lungs of ten-week-old pigs. Pediatr Pulmonol. 1997;23(3):205–211 [DOI] [PubMed] [Google Scholar]
- 41.Wu JM, Yeh TF, Wang JY, et al. The role of pulmonary inflammation in the development of pulmonary hypertension in newborn with meconium aspiration syndrome (MAS). Pediatr Pulmonol Suppl. 1999;18:205–208 [PubMed] [Google Scholar]
- 42.van Ierland Y, de Beaufort AJ. Why does meconium cause meconium aspiration syndrome? Current concepts of MAS pathophysiology. Early Hum Dev. 2009;85(10):617–620 [DOI] [PubMed] [Google Scholar]
- 43.Polglase GR, Hooper SB, Gill AW, et al. Intrauterine inflammation causes pulmonary hypertension and cardiovascular sequelae in preterm lambs. J Appl Physiol. 2010;108(6):1757–1765 [DOI] [PubMed] [Google Scholar]
- 44.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8(8):443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]