Abstract
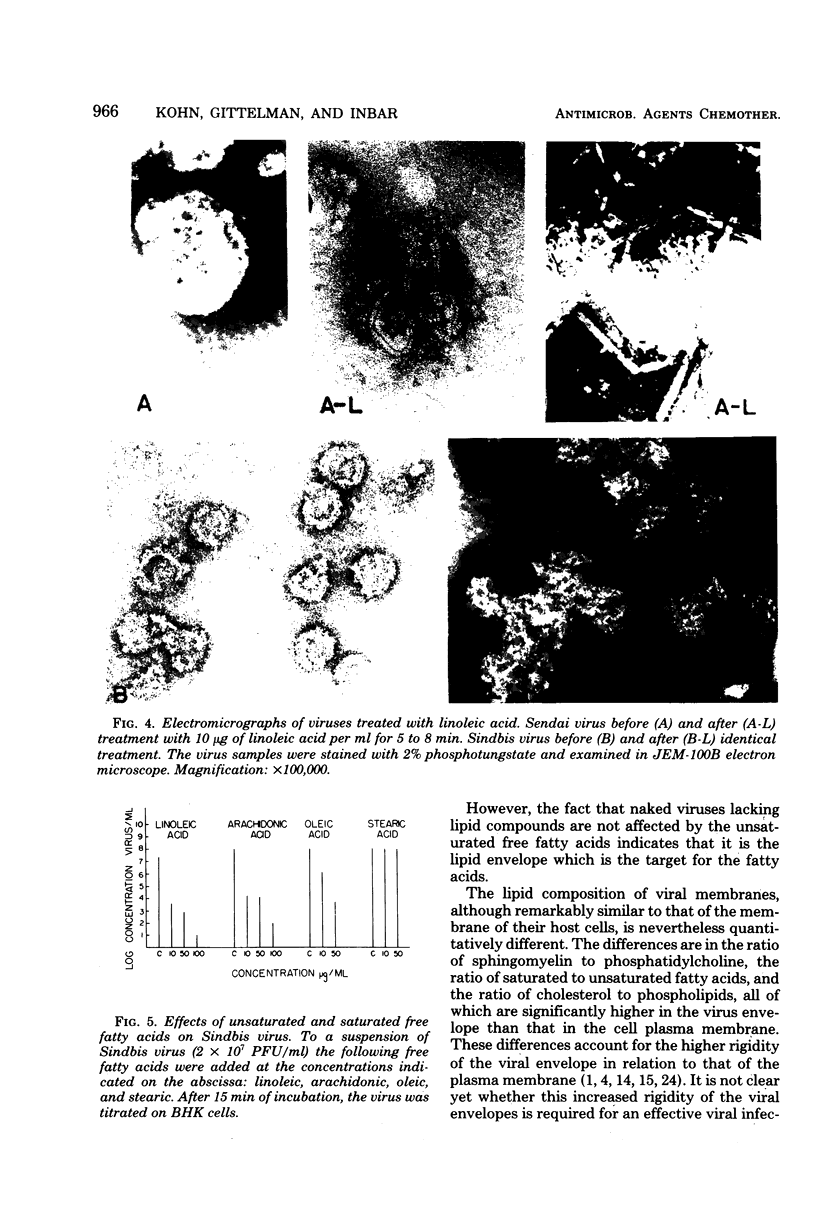
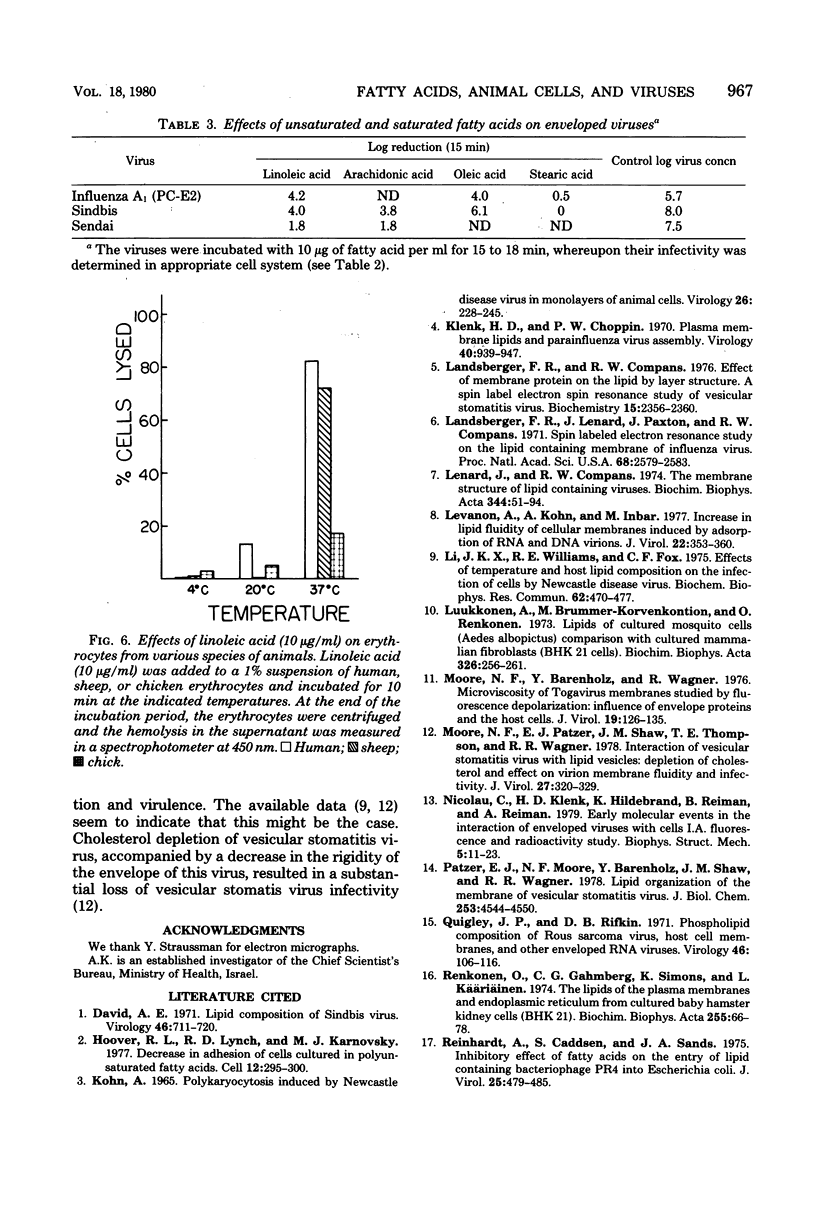
Essential unsaturated fatty acids such as oleic, linoleic, or arachidonic were incorporated into the phospholipids of animal cells and induced in them a change in the fluidity of their membranes. Exposure of enveloped viruses such as arbo-, myxo-, paramyxo-, or herpesviruses to micromolar concentrations of these fatty acids (which are not toxic to animal cells) caused rapid loss of infectivity of these viruses. Naked viruses such as encephalomyocarditis virus, polio virus or simian virus 40 were not affected by incubation with linoleic acid. The loss of infectivity was attributed to a disruption of the lipoprotein envelope of these virions, as observed in an electron microscope.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- David A. E. Lipid composition of Sindbis virus. Virology. 1971 Dec;46(3):711–720. doi: 10.1016/0042-6822(71)90073-0. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Lynch R. D., Karnovsky M. J. Decrease in adhesion of cells cultured in polyunsaturated fatty acids. Cell. 1977 Sep;12(1):295–300. doi: 10.1016/0092-8674(77)90207-0. [DOI] [PubMed] [Google Scholar]
- KOHN A. POLYKARYOCYTOSIS INDUCED BY NEWCASTLE DISEASE VIRUS IN MONOLAYERS OF ANIMAL CELLS. Virology. 1965 Jun;26:228–245. doi: 10.1016/0042-6822(65)90050-4. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Plasma membrane lipids and parainfluenza virus assembly. Virology. 1970 Apr;40(4):939–947. doi: 10.1016/0042-6822(70)90140-6. [DOI] [PubMed] [Google Scholar]
- Landsberger F. R., Compans R. W. Effect of membrane protein on lipid bilayer structure: a spin-label electron spin resonance study of vesicular stomatitis virus. Biochemistry. 1976 Jun 1;15(11):2356–2360. doi: 10.1021/bi00656a017. [DOI] [PubMed] [Google Scholar]
- Landsberger F. R., Lenard J., Paxton J., Compans R. W. Spin-labeled electron spin resonance study of the lipid-containing membrane of influenza virus. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2579–2583. doi: 10.1073/pnas.68.10.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J., Compans R. W. The membrane structure of lipid-containing viruses. Biochim Biophys Acta. 1974 Apr 8;344(1):51–94. doi: 10.1016/0304-4157(74)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanon A., Kohn A., Inbar M. Increase in lipid fluidity of cellular membranes induced by adsorption of RNA and DNA virions. J Virol. 1977 May;22(2):353–360. doi: 10.1128/jvi.22.2.353-360.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. K., Williams R. E., Fox C. F. Effects of temperature and host lipid composition on the infection of cells by Newcastle disease virus. Biochem Biophys Res Commun. 1975 Jan 20;62(2):470–477. doi: 10.1016/s0006-291x(75)80162-8. [DOI] [PubMed] [Google Scholar]
- Luukkonen A., Brummer-Korvenkontio M., Renkonen O. Lipids of cultured mosquito cells (Aedes albopictus). Comparison with cultured mammalian fibroblasts (BHK 21 cells). Biochim Biophys Acta. 1973 Nov 29;326(2):256–261. doi: 10.1016/0005-2760(73)90251-8. [DOI] [PubMed] [Google Scholar]
- Moore N. F., Barenholz Y., Wagner R. R. Microviscosity of togavirus membranes studied by fluorescence depolarization: influence of envelope proteins and the host cell. J Virol. 1976 Jul;19(1):126–135. doi: 10.1128/jvi.19.1.126-135.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N. F., Patzer E. J., Shaw J. M., Thompson T. E., Wagner R. R. Interaction of vesicular stomatitis virus with lipid vesicles: depletion of cholesterol and effect on virion membrane fluidity and infectivity. J Virol. 1978 Aug;27(2):320–329. doi: 10.1128/jvi.27.2.320-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolau C., Klenk H. D., Hildenbrand K., Reimann B., Reimann A., Bauer H. Early molecular events in the interaction of enveloped viruses with cells. I. A fluorescence and radioactivity study. Biophys Struct Mech. 1979 Mar 21;5(1):11–23. doi: 10.1007/BF00535769. [DOI] [PubMed] [Google Scholar]
- Patzer E. J., Moore N. F., Barenholz Y., Shaw J. M., Wagner R. R. Lipid organization of the membrane of vesicular stomatitis virus. J Biol Chem. 1978 Jul 10;253(13):4544–4550. [PubMed] [Google Scholar]
- Quigley J. P., Rifkin D. B., Reich E. Phospholipid composition of Rous sarcoma virus, host cell membranes and other enveloped RNA viruses. Virology. 1971 Oct;46(1):106–116. doi: 10.1016/0042-6822(71)90010-9. [DOI] [PubMed] [Google Scholar]
- Reinhardt A., Cadden S., Sands J. A. Inhibitory effect of fatty acids on the entry of the lipid-containing bacteriophage PR4 into Escherichia coli. J Virol. 1978 Feb;25(2):479–485. doi: 10.1128/jvi.25.2.479-485.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkonen O., Gahmberg C. G., Simons K., Käriäinen L. The lipids of the plasma membranes and endoplasmic reticulum from cultured baby hamster kidney cells (BHK21). Biochim Biophys Acta. 1972 Jan 17;255(1):66–78. doi: 10.1016/0005-2736(72)90008-9. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. S., Yanovich S., Inbar M., Strominger J. L. Translocation of a hydrocarbon fluorescent probe between Epstein-Barr virus and lymphoid cells: an assay for early events in viral infection. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5076–5080. doi: 10.1073/pnas.75.10.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands J. A., Auperin L. D., Reinhardt A. Enveloped virus inactivation by fatty acid derivatives. Antimicrob Agents Chemother. 1979 Jan;15(1):134–136. doi: 10.1128/aac.15.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands J. A., Reinhardt A., Auperin D., Landin P. Inhibition of entry of the lipid-containing bacteriophage PR4 by fatty acid derivatives. J Virol. 1979 Jan;29(1):413–416. doi: 10.1128/jvi.29.1.413-416.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands J., Auperin D., Snipes W. Extreme sensitivity of enveloped viruses, including herpes simplex, to long-chain unsaturated monoglycerides and alcohols. Antimicrob Agents Chemother. 1979 Jan;15(1):67–73. doi: 10.1128/aac.15.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Gaffney B. J. Effect of the viral proteins on the fluidity of the membrane lipids in Sindbis virus. J Mol Biol. 1974 Dec 5;90(2):343–358. doi: 10.1016/0022-2836(74)90378-7. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Microviscosity parameters and protein mobility in biological membranes. Biochim Biophys Acta. 1976 Apr 16;433(1):133–149. doi: 10.1016/0005-2736(76)90183-8. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Bister K. 13-C nuclear magnetic resonance studies on the lipid organization in enveloped virions (vesicular stomatitis virus). Biochemistry. 1975 Jul;14(13):2841–2847. doi: 10.1021/bi00684a008. [DOI] [PubMed] [Google Scholar]
- Tanford C. The hydrophobic effect and the organization of living matter. Science. 1978 Jun 2;200(4345):1012–1018. doi: 10.1126/science.653353. [DOI] [PubMed] [Google Scholar]
- Tiffany J. M., Blough H. A. Myxovirus envelope proteins: a directing influence on the fatty acids of membrane lipids. Science. 1969 Feb 7;163(3867):573–574. doi: 10.1126/science.163.3867.573. [DOI] [PubMed] [Google Scholar]
- Williams R. E., Wisnieski B. J., Rittenhouse H. G., Fox C. F. Utilization of fatty acid supplements by cultured animal cells. Biochemistry. 1974 Apr 23;13(9):1969–1977. doi: 10.1021/bi00706a029. [DOI] [PubMed] [Google Scholar]