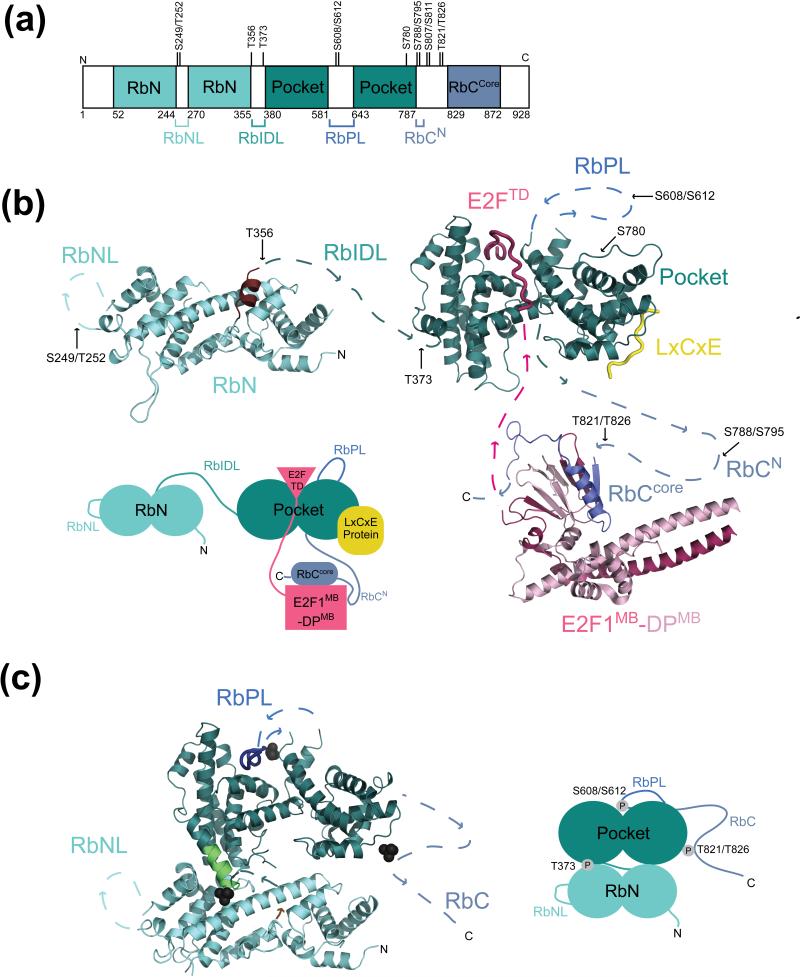
Figure 1.
Retinoblastoma protein (Rb) structure, phosphorylation sites, and protein interactions. (a) Domain organization and location of Cdk phosphorylation sites. The structured domains, which are colored, include the N-terminal domain (RbN) and the pocket domain. In contrast, several regions are intrinsically disordered including two large loops in RbN (RbNL) and the pocket (RbPL), an interdomain linker (RbIDL), and parts of the C-terminal domain (RbC). (b) Model of unphosphorylated Rb from crystal structures of individual domains and complexes with E2F and an LxCxE peptide (PDB codes: 2QDJ, 1GUX, 1N4M, and 2ACE). Unstructured loops and linkers connecting the structured domains are represented as broken lines. Approximate locations of phosphorylation sites are indicated. The Rb domains are colored as in (a); E2F is in pink, and the LxCxE peptide is in yellow. The C-terminal helix of RbN, which becomes disordered upon T356 phosphorylation is colored brown. (c) Model of phosphorylated Rb from crystal structures (PDB codes: 4ELJ and 4ELL). Only phosphates that are known to promote intramolecular interactions (T373, S608/S612, and T821/T826) are shown. T821/T826 induces binding of RbC to the pocket domain at the LxCxE site, although there is no high-resolution structure detailing this interaction. The N-terminal helix of the pocket domain that is nucleated by T373 phosphorylation is shown in green.