Fig. 1.
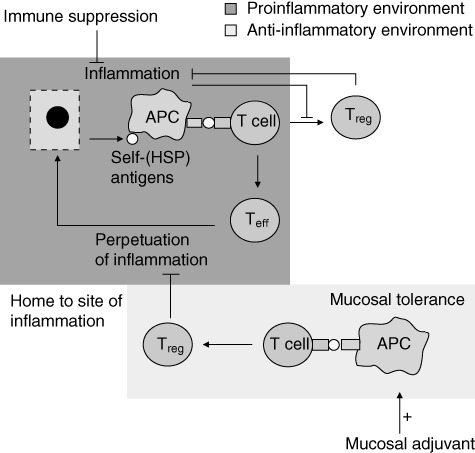
The dual role of heat shock proteins (HSPs), inducing proinflammatory effector T cells (Teff) or tolerogenic/regulatory T cells (Treg), is influenced by the inflammation status of the tissues. At the site of inflammation, self-HSP antigens are released from damaged cells. These antigens are presented by activated antigen-presenting cells (APC) to T cells. In a proinflammatory environment this results in predominantly Teff cells, contributing to perpetuation of inflammation. T cells induced via the mucosal route are directed by the anti-inflammatory environment towards a predominantly tolerogenic response (Treg) (mucosal tolerance induction). The mucosally induced antigen-specific T cells consist of multiple kinds of regulatory cells and which are thought to migrate to the site of inflammation as their cognate antigen (e.g. HSP) is expressed there. At the site of inflammation, these antigen-specific Tregs skew the proinflammatory T cell response towards an anti-inflammatory phenotype by cytokine release-like interleukin (IL)-10 or cell–cell contact. Mucosal adjuvant can enhance peptide presentation by APCs at the site of tolerance induction, enlarging the pool of Treg formed after mucosal tolerance induction. The inflammatory environment hampers the development of (self-HSP-specific) T cells. Generalized immune suppressive therapy reduces inflammation, creating a more favourable environment for the development of Treg. Combination therapy of antigen-specific mucosal tolerance induction with immune suppressive therapy could therefore enhance efficacy of peptide specific immunotherapy.
