Fig. 2.
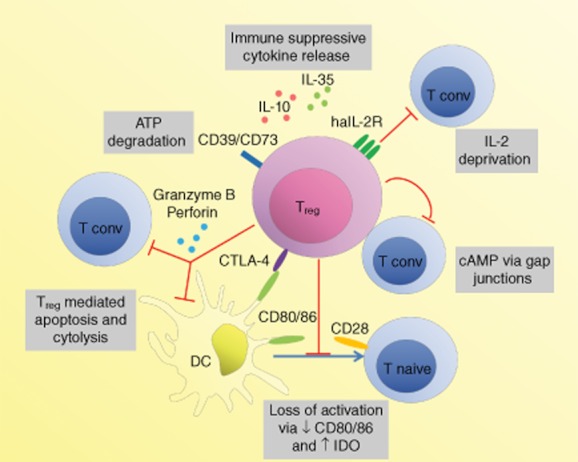
Mechanisms of regulatory T cell-mediated suppression. Regulatory T cells (Tregs) can mediate suppression through their effects on dendritic cells (DCs), such as physical inhibition of interaction between DCs and conventional T cells (Tconv) or deprivation of co-stimulation or soluble factors. Cytotoxic T lymphocyte-associated antigen (CTLA)-4 on Tregs interacts with CD80 and CD86 on DC with high affinity. In addition to inhibiting co-stimulation via CD28 on Tconv, this can lead to down-regulation of CD80 and CD86 expression on the DC (either through transendocytosis or transcriptional regulation), thereby further reducing their co-stimulatory capacity and Tconv activation and proliferation. It can also lead to up-regulation of indolamine 2,3-dioxygenase (IDO) in DC, which can mediate suppression of Tconv by tryptophan depletion and apoptosis through tryptophan catabolites. Perforin and granzyme B from Tregs induces apoptosis and cytolysis of both Tconv and DC. Deprivation of interleukin (IL)-2 via the high-affinity IL-2 receptor (haIL-2R, consisting of a CD25, CD122, CD132 complex) and adenosine triphosphate (ATP) through CD39 and CD73 can lead to the inhibition of Tconv activation or T cell apoptosis. The cytokines IL-10 and IL-35 can mediate suppression, and IL-35 can also act as an autocrine Treg growth factor. While secreted TGF-β induces IDO in DCs, how much the cytokine contributes to Treg-mediated suppression is under debate. Cyclic adenosine monophosphate (cAMP) transferred to Tconv via gap junctions inhibits their IL-2 production and subsequent proliferation. A number of other suppression mechanisms have been proposed. The relative contributions of these processes remain to be determined. Generally, spatiotemporal context may determine which of these mechanisms dominate. CD39: nucleoside triphosphate diphosphohydrolase; CD73: ecto-5′-nucleotidase.
