Abstract
Cardiovascular disease remains the leading cause of death for both men and women. Hypertension is less prevalent in young women compared with young men, but menopausal women are at greater risk for hypertension compared with men of similar age. Despite these risks, women do not consistently receive first line treatment for the early stages of hypertension, and the greater morbidity in menopause reflects this neglect. This review focuses on ovarian hormone effects on the cardiovascular and water regulatory systems that are associated with blood pressure control in women. The study of ovarian hormones within young women is complex because these hormones fluctuate across the menstrual cycle, and these fluctuations can complicate conclusions regarding sex differences. To better isolate the effects of oestrogen and progesterone on the cardiovascular and water regulation systems, we developed a model to transiently suppress reproductive function followed by controlled hormone administration. Sex differences in autonomic regulation of blood pressure appear related to ovarian hormone exposure, and these hormonal differences contribute to sex differences in hypertension and orthostatic tolerance. Oestrogen and progesterone exposure are also associated with plasma volume expansion, and a leftward shift in the osmotic operating point for body fluid regulation. In young, healthy women, the shift in osmoregulation appears to have only a minor effect on overall body water balance. Our overarching conclusion is that ovarian hormone exposure is the important underlying factor contributing to differences in blood pressure and water regulation between women and men, and within women throughout the lifespan.
Nina Stachenfeld's research interests focus on sex hormone effects on autonomic control of blood pressure, body fluid regulation and temperature regulation. She worked with the late Ethan R. Nadel and Gary W. Mack for her Post Doctoral training at the John B. Pierce Laboratory, where she has continued her career. Dr Stachenfeld is also a member of the faculty at the Yale School of Medicine in the Departments of Obstetrics, Gynecology and Reproductive Sciences and the Yale School of Public Health. Megan Wenner's research interests focus on ageing and ovarian hormone effects on the mechanisms regulating blood pressure in women. She completed her Post Doctoral training at the John B. Pierce Laboratory with Dr Stachenfeld. Dr Wenner is currently an Assistant Professor in the Department of Kinesiology and Applied Physiology at the University of Delaware.
 |
Introduction
Cardiovascular disease is the leading cause of death in American men and women (Lloyd-Jones et al. 2010). The prevalence of hypertension in adults under 45 years of age is lower in women compared with men but is greater in postmenopausal women relative to men over 55 years (Roger et al. 2011). Across the lifespan, hypertension risk is approximately 29% and 31% for US women and men, respectively (Keenan & Rosendorf, 2011). Despite these data, there has been a bias assuming women are protected from hypertension. Until recent years women were excluded from landmark studies that set the standards for detection and treatment of heart disease and hypertension, and women have not consistently received first line treatment for the early stages of hypertension. We believe this neglect is reflected in higher cardiovascular disease and hypertension morbidity in older women compared with men with similar signs and symptoms. Within this review consistent with WHO definitions, we use the word ‘sex’ to define biological and physical characteristics when discussing differences between men and women (WHO, 2012). Although gender differences play an important role in human health, gender (which generally refers to socially constructed roles and attributes that society assigns to men and women (WHO, 2012)) is beyond the scope of this review.
Focus of this review
The primary functions of oestrogens and progesterone are in reproduction. However, these hormones also influence the integrated cardiovascular, neural and hormonal systems that control blood pressure, blood volume, thirst, fluid intake, and renal water and sodium regulation. Although we fully recognize that sex differences are not limited to sex hormone exposure, the overarching hypothesis for this review is that the potent effects of ovarian hormone exposure on autonomic function and osmoregulation are the primary factors contributing to the sex differences in blood pressure and water balance in humans. These ovarian hormones have complex and, at times, opposing physiological effects on the cardiovascular and water regulation systems.
Sex differences versus hormone exposure
Differences between men and women that are related to sex hormone exposure are exaggerated or minimized at different points in a woman's menstrual cycle because of the large fluctuations in hormone exposure in women across the cycle (Fig. 1A and B). For example, men and women differ in osmotic regulation of arginine vasopressin (AVP) during the early follicular phase (days 1–6) of the menstrual cycle (when oestradiol and progesterone exposure are lowest in women), but this difference is not apparent when men are compared with women in the mid-luteal phase (∼day 21, when oestrogen and progesterone exposure is high in women) (Stachenfeld et al. 2001) (see Fig. 1B). A similar trend for this pattern in sex differences revealed differences in sympathetic nerve activity during upright tilt in the follicular phase but not in the luteal phase (Fig. 2 (Fu et al. 2009)). This is an important point to bear in mind because the preponderance of physiological testing in studies that include both men and women are conducted when women are in the early follicular phase with the intent of reducing variability between the sexes.
Figure 1. Plasma fluctuations of hormones and gonadotropins over a normal 28 day menstrual cycle.
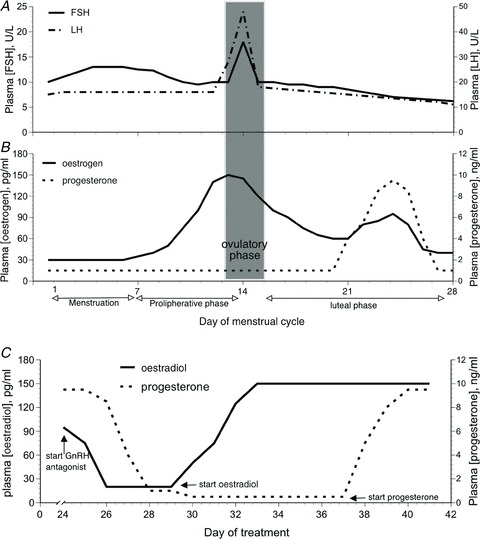
A, follicle stimulating hormone (FSH) and luteinizing hormone (LH); B, oestrogens and progesterone. C, changes in 17 β-oestradiol and progesterone during gonadotropin releasing hormone (GnRH) antagonist administration followed by 17 β-oestradiol and progesterone administration.
Figure 2. Muscle sympathetic nerve activity (MSNA) burst frequency (A) and total activity (B) responses during a graded upright tilt in men and women during the early follicular phase (oestrogen and progesterone are low) and the mid-luteal phase (oestrogen and progesterone are high).
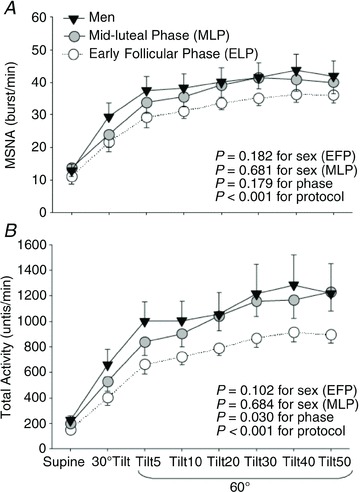
Data are mean ± SEM. Tilt5, Tilt10, Tilt20, Tilt30, Tilt40 and Tilt45 are 5, 10, 20, 30, 40 and 45 min after 60 deg upright tilt. From Fu et al. (2009) with permission.
Considering reproductive hormone exposure in women
Another difficulty with the study design in which experiments are conducted near menstruation is that women are tested during the first 7 days of a 28 day cycle (Fig. 1A and B), which accounts for one fourth of their reproductive life. Moreover, the gonadotropin follicle stimulating hormone (FSH) changes in the early part of the menstrual cycle (Fig. 1A) so this period is not hormonally stable even though the ovarian hormones are not changing. An alternative to early follicular phase testing is studying women during oral contraceptive pill (OCP) administration thereby controlling reproductive hormone exposure. Recent data from the US Department of Health and Human Services indicate that >90% of US women have used hormonal contraception at some time in their life (Mosher & Jones, 2010), so these studies provide clinically relevant data. However, this study design also has limitations, beginning with the problem of comparing women who are taking exogenous hormones that increase hormone exposure above that of endogenous levels at any point in the menstrual cycle. Another weakness of this study design is that progestins in OCP have androgenic properties relative to endogenous progesterone (Speroff et al. 1999), and androgens alter peripheral vasodilatation (El-Mas et al. 2001, 2002; Sokolnicki et al. 2007; Wenner et al. 2011a) and blood pressure (Roesch & Keller-Wood, 1997; Reckelhoff & Granger, 1999) so can impact studies examining blood pressure regulation. To further compound the challenges of this study design, these studies often use the week of placebo pills, or ‘low hormone’ phase as a basis of comparison to the OCP. This week is not a consistent period of low hormone exposure because progestin and oestradiol metabolites from OCP remain in tissue (for variable lengths of time and concentrations among women), so exposure is not reliably low during the placebo week. Finally, withdrawal of the OCP can induce endogenous production of oestradiol in the placebo or so called ‘low hormone’ phase (Speroff et al. 1999; van Heusden & Fauser, 1999; Creinin et al. 2002; Schlaff et al. 2004), and these increases are not consistently reflected in blood.
To isolate individual effects of oestradiol or progesterone on physiological systems, we developed a model to temporarily suppress the menstrual cycle (and thus reproductive hormones) using either a gonadotropin releasing hormone (GnRH) agonist (leuprolide acetate, Lupron) or antagonist (ganirelix acetate, Antagon). Leuprolide, the agonist, has greater receptor binding and decreased degradation compared with endogenous GnRH, so is a potent inhibitor of gonadotropin secretion. When leuprolide is given continuously, the hypothalamic–pituitary–ovarian axis is down-regulated, with internalization and uncoupling of the GnRH receptors at the pituitary level. Thus, following an initial stimulation, chronic GnRH agonist administration suppresses FSH-related steroidogenesis, leading to low or undetectable oestrogen and progesterone concentrations within 14 days (Halmos et al. 1996; Taylor et al. 2010). Ganirelix, the GnRH antagonist, is derived from native GnRH with substitutions at positions 1, 2, 3, 6, 8 and 10 and competitively blocks the GnRH receptors on the pituitary gonadotroph inducing a rapid, reversible suppression of gonadotropin secretion (Oberye et al. 1999a,b; Olivennes, 2006). In eumenorrheic women, ganirelix administration suppresses oestrogens and progesterone to postmenopausal levels after 48 h of administration (Fig. 1C). During both leuprolide and ganirelix administration we can isolate the effects of oestrogens and progesterone in young women by selectively adding the natural forms of these hormones back, thus permitting causal inferences about their functions on the system targeted for study. This method has its own weakness in that it is more invasive than other hormone interventions in humans, although it is well tolerated.
Autonomic control of blood pressure
Sex differences in autonomic control
Although studies report no differences in baroreflex sensitivity between men and women at rest (Tank et al. 2005) or during orthostatic stress (Fu et al. 2009), young women have lower resting blood pressure compared with young men (Christou et al. 2005), and mechanisms regulating blood pressure differ between men and women (Charkoudian et al. 2005, 2006a; Hart et al. 2009; Joyner et al. 2010; Hart et al. 2011). Young women have lower blood pressure reductions during gangiolic blockade and exaggerated blood pressure responses to phenylephrine infusions compared with men (Christou et al. 2005). These findings suggest both lower sympathetic support of blood pressure and reduced baroreflex buffering in women relative to men, which may explain their lower resting blood pressure.
In young men, muscle sympathetic nerve activity (MSNA) is positively correlated with total peripheral resistance (TPR), and is inversely correlated with cardiac output (CO), indicating low CO buffers increases in sympathetic nerve activity to maintain blood pressure (Charkoudian et al. 2005). In contrast, neither TPR nor CO are related to MSNA in young women (Hart et al. 2009). Thus, other mechanisms such as vascular responsiveness and β-adrenergic balance to α-adrenergic vasoconstriction may play a more prominent role in blood pressure regulation in women than in men (Kneale et al. 2000; Hart et al. 2011). Importantly, in the studies described above examining sex differences, the timing of the testing in relation to endogenous hormone levels was either not controlled (Christou et al. 2005), with some of the women taking OCP (Tank et al. 2005), and others tested during the early follicular phase or during placebo phase of OCP (Hart et al. 2009). Thus it is difficult to determine the contribution of ovarian hormones on these sex difference in blood pressure regulation. For a recent review of mechanisms related to sex differences in blood pressure regulation see Hart et al. (2012).
Oestrogens and progesterone impact on autonomic control of blood pressure
We propose that ovarian hormone exposure can explain the variability in blood pressure regulation control systems between men and women. Resting sympathetic outflow is greater during the luteal phase – when both oestrogens and progesterone are elevated – compared with the early follicular phase of the menstrual cycle – when both oestrogens and progesterone are low (Minson et al. 2000a; Carter et al. 2009b; Fu et al. 2009). During an orthostatic challenge, total MSNA (which takes into account the area under the MSNA burst) is also greater in the mid-luteal versus early follicular phase (Carter et al. 2009b; Fu et al. 2009) (Fig. 2). Despite these findings, changes in baroreflex sensitivity during the menstrual cycle are conflicting. For example, during a modified Oxford protocol, sympathetic baroreflex sensitivity is greater in the mid-luteal compared with the early follicular phase (Minson et al. 2000a), but sympathetic baroreflex sensitivity is similar across the early follicular and mid-luteal menstrual phases during orthostasis (Carter et al. 2009b; Fu et al. 2009). An explanation for the discrepancy between these studies is not obvious, but may be explained by the method of assessing baroreflex function. While both studies used the slope of the linear regression of MSNA versus diastolic blood pressure, Minson et al. (2000a) examined these responses during bolus injections of sodium nitroprusside and phenylephrine (modified Oxford technique), while the Fu et al. (2009) and Carter et al. (2009b) studies determined baroreflex sensitivity from spontaneous fluctuations in MSNA and DBP during LBNP or tilt table testing. The modified Oxford technique allows for determination of the MSNA–DBP relationship over a wide range of blood pressure, while the spontaneous method examines specifically the smaller physiological range. Similarly, discrepancies exist with regard to changes in baroreflex control of heart rate across the menstrual cycle, with some studies reporting no change in baroreflex control of heart rate across the menstrual cycle (Minson et al. 2000a; Cooke et al. 2002) or an increase in baroreflex control of heart rate in the pre-ovulatory phase compared with the early follicular phase (Tanaka et al. 2003). Nonetheless, while there are some inconsistencies across investigations, together these data suggest that sex hormones impact sympathetic neural control of baroreflex function.
Based on the studies described above, it is unclear whether oestrogens or progesterone exposure is most important to the changes in baroreflex function across the menstrual cycle. Baroreflex control of heart rate increases near ovulation when oestrogen peaks (Tanaka et al. 2003; Brooks et al. 2012), indicating an important role for oestrogens. Conversely, in rats baroreflex function is suppressed through the neurohormone 3α-hydroxydihydroprogesterone via GABAnergic influences (Brooks et al. 2010; Laiprasert, 1998 no. 7656}. The opposing influences of oestrogens and progesterone on baroreflex function make understanding the impact of hormones on the baroreflex within women challenging, and may also have led to the discrepancies among the various studies. Studies that isolate these hormones during testing using the GnRH antagonist–hormone administration method will help to isolate the particular hormone involved in altering baroreflex function in young women.
There remains some controversy regarding the impact of OCP on baroreflex function. A number of studies have demonstrated that resting MSNA and plasma noradrenaline (norepinephrine) are unaffected by OCP phase (Minson et al. 2000b; Carter et al. 2009a). Using pharmacological perturbation of blood pressure (modified Oxford), Minson et al. (2000b) demonstrated suppressed sympathetic and cardiovagal baroreflex sensitivity during the OCP active pill phase compared with a placebo phase. In contrast, Carter et al. (2009a) found no effect of OCP on MSNA responses or sympathetic baroreflex sensitivity during lower body negative pressure (Carter et al. 2009a). The conflicting findings between these studies are difficult to reconcile, but may be related to methodological differences such as modified Oxford versus the LBNP manipulation of blood pressure as discussed earlier. In addition, these studies used different types and doses of OCP, so the variability in the type and magnitude of hormone exposure also makes them difficult to compare, as the oestradiol–progestin composition of the OCPs, both within and across studies, were not standardized (Minson et al. 2000b; Carter et al. 2009a). Furthermore, because all studies used OCPs with combined oestradiol and progestin, the independent effects of oestradiol and progestins on baroreflex function remain unclear, and the actions of these hormones can oppose each other. Despite these challenges, these studies using OCP did support a role of ovarian hormone exposure on autonomic control of blood pressure in women. Moreover, as mentioned earlier, OCPs have been used by ∼90% of women, so more consistent OCP studies will be essential to provide data on cardiovascular control mechanisms during OCP administration.
Menopause and ageing effects on autonomic function
Young women tend to have lower blood pressure than men but lose this protection as they age and enter menopause. In menopause women lose ovarian function, associated with a permanent cessation of menstruation and low oestrogen and progesterone exposure beginning between the ages of 50–55 years. Cardiovascular disease, stroke and hypertension prevalence in menopausal women surpasses that of men of similar age, and approximately 75% of postmenopausal women become hypertensive (Roger et al. 2011). The direct mechanisms involved in changing blood pressure regulation in menopausal women have not been definitively determined but the sympathetic nervous system likely plays an important role. Although in young healthy men and women MSNA is unrelated to resting blood pressure, there appears to be a direct relationship between MSNA and blood pressure in older humans, and this relationship is especially strong in women (Narkiewicz et al. 2005). Although sympathetic activity is lower in young women compared with young men, MSNA increases with age in both sexes, and some reports find similar MSNA in older men and women (Matsukawa et al. 1998; Narkiewicz et al. 2005), while other reports find the greater MSNA in young men relative to women continued into their later years (Ng et al. 1993). Although these studies report slightly different findings with regard to MSNA and sex into older age, they all indicate a rise in blood pressure in older women, indicating the cardiovascular system in women becomes more sensitive to sympathetic input as they age (Ng et al. 1993; Matsukawa et al. 1998; Narkiewicz et al. 2005). The mechanism for the changes in sympathetic nervous system in function in women has not yet been confirmed, but may be a direct result of oestrogen withdrawal because oestrogen administration decreases noradrenaline spillover (Sudhir et al. 1997), MSNA (Vongpatanasin et al. 2001) and enhances sympathetic baroreflex sensitivity (Hunt et al. 2001) in menopausal women.
Vascular responsiveness
Sex differences in vascular responsiveness
Sex differences in blood pressure control are also likely related to differences in vascular responsiveness, and vascular reactivity differs between men and women. Brachial artery infusions of the β-2 agonist albuterol induce greater increases in forearm blood flow, and plasma noradrenaline infusions with and without the β-antagonist propranolol, induce a lesser vasoconstriction of forearm blood vessels in women compared with men (Kneale et al. 2000). Moreover, the vasoconstrictor response to noradrenaline is lower in women compared with men although blocking the β-receptors with propranolol removes the sex differences, suggesting that enhanced β-2 receptor vasodilatation in women attenuates the adrenergic vasoconstrictor response (Hart et al. 2011). These studies indicate a greater β-adrenergic (Kneale et al. 2000; Hart et al. 2011) and lower α-adrenergic (Schmitt et al. 2010) support of blood pressure in women compared with men. Furthermore, women tend to have greater vasodilatory responses during reactive hyperaemia compared with men (Hashimoto et al. 1995; Levenson et al. 2001).
Oestrogens and progesterone effects on vascular responsiveness
Consistent with our overall hypothesis, the differences in vascular responsiveness between men and women are likely mediated by ovarian hormones. Oestrogens appear to have a direct effect on the vasculature. Oestrogen receptors are found on the endothelium and enhance nitric oxide bioavailability (Orshal & Khalil, 2004) so probably contribute to sex differences in vascular responsiveness. High endogenous oestrogen exposure is associated with increases in flow-mediated vasodilatation (FMD), an index of endothelial function in humans (Hashimoto et al. 1995; Williams et al. 2001; Adkisson et al. 2010). Similarly, administering exogenous oestradiol enhances FMD in young women (Meendering et al. 2008; Miner et al. 2011) and attenuates vasoconstrictor responses to noradrenaline (Sudhir et al. 1997). As proposed earlier, data in rats suggest the attenuated vasoconstrictor response associated with oestradiol exposure appears to be a result of reduced α- concomitant with greater β-adrenergic receptor actions (Ferrer et al. 1996; Zhang & Davidge, 1999). In ovariectomized rat mesenteric arteries, exposure to physiological levels of oestradiol attenuated vasoconstriction during phenylephrine infusion (Zhang & Davidge, 1999), and enhanced the vasodilatory responses to isoproterenol infusion (Ferrer et al. 1996). Thus, the mechanisms contributing to the greater vascular relaxation by oestradiol include enhanced NO bioavailability, greater β-adrenergic and lower α-adrenergic actions.
Proges terone receptors have also been identified in human endothelial cells of the aorta, internal carotid artery and coronary arteries (Lin et al. 1982; Ingegno et al. 1988; Lee et al. 1997), supporting the argument that progesterone has also direct effects on the vasculature. Progesterone has both vasodilatory and vasoconstrictive effects in the vasculature depending on location of the vessel and level of exposure. Indeed, progesterone at physiological levels can inhibit the production of endothelin-1 in bovine aortic endothelial cells (Morey et al. 1997), but at supraphysiological levels inhibits endothelium-independent relaxation by blocking calcium channels in vascular smooth muscle (Jiang et al. 1992; Perusquia et al. 1996). Furthermore, physiological progesterone exposure diminishes the vasodilatory effects of oestradiol on FMD during GnRH suppression (Miner et al. 2011). Combined oestradiol and progesterone administration enhances peripheral cutaneous vasoconstrictor response to cutaneous noradrenaline infusions in women with high orthostatic tolerance, but combined administration of these same ovarian hormones does not influence vasoconstrictor responses to noradrenaline infusions in women with low orthostatic tolerance (Wenner et al. 2011b). Thus, low sensitivity to progesterone-mediated vasoconstriction provides less sympathetic support for blood pressure in women with low orthostatic tolerance during orthostatic stress.
Ovarian hormones and water regulation
Overview of water regulation
Fluid regulatory systems are sensitive to stimuli arising from water deficits or increased blood sodium, tonicity or osmolality in the extracellular fluid space or plasma. Arginine vasopressin (AVP; or antidiuretic hormone), synthesized in the cell bodies of nuclei located in the anterior hypothalamus, is a powerful vasoconstrictor and regulates renal free water clearance. Axons from the anterior hypothalamus project into the posterior pituitary where AVP is stored and released in response to stimulation of central osmoreceptors. Arginine vasopressin is sensitive to increases in plasma osmolality as small as 5 mosmol (kg H2O)-1 (2–3%), leading to an immediate and linear AVP response (Calzone et al. 2001; Stachenfeld et al. 2001; Stachenfeld & Keefe, 2002). Thirst and AVP are also sensitive to volume stimuli via peripheral baroreceptors, but require plasma volume changes of ∼10% to trigger AVP release or thirst sensation in humans. Thus, to determine sex differences in, or sex hormone effects on, osmotic regulation of AVP in humans, we examined the linear slope and intercept of the P[AVP]:plasma osmolality (POsm) and thirst:POsm linear relationships during dehydration or a 2 h hypertonic saline infusion between men and women under different hormone conditions. Important for this review, the hypothalamic nuclei that produce AVP contain oestrogen receptors (Heritage et al. 1980; Sar & Stumpf, 1980).
Sex differences in water regulation
Neuron activity and size in hypothalamic nuclei responsible for AVP release are greater in men compared with women (Ishunina & Swaab, 1999). Resting P[AVP] is greater in men than women when women are in the early follicular phase of their menstrual cycle, but not in the mid-luteal phase (Claybaugh et al. 2000; Stachenfeld et al. 2001). (Thus, the discovery of sex differences in these systems is dependent on the phase of the women's menstrual cycle in which the studies take place.) Men have greater AVP sensitivity but lower water turnover in response to hypertonic saline infusion compared with women regardless of menstrual cycle phase (Claybaugh et al. 2000; Stachenfeld et al. 2001). Men also have higher nocturnal P[AVP] despite similar urine osmolality compared with women, suggesting greater renal sensitivity to AVP in women compared with men at night (Hvistendahl et al. 2007). During hypertonic saline infusion, osmotic threshold for AVP release is lower in men compared with women during the early follicular but not during the mid-luteal phase of the menstrual cycle (Stachenfeld et al. 2001) (Fig. 3). Indeed P[AVP] doubled during hypertonic saline infusion, with no effect on free water clearance, indicating lower renal sensitivity to AVP in men versus women (in the luteal phase) (Stachenfeld et al. 2001). Thus androgens may increase AVP sensitivity whereas oestrogens lower the POsm threshold for AVP release (Stachenfeld et al. 1998, 2001; Stachenfeld & Keefe, 2002).
Figure 3. Mean plasma arginine vasopressin concentration (P[AVP]) in response to increases in POsm during hypertonic saline infusion in the early follicular and mid-luteal phases in women and in men.
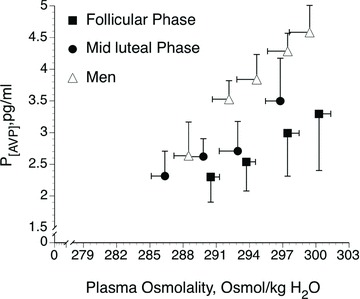
Data are mean ± SEM. From Stachenfeld et al. (2001).
Oestrogen and progesterone effects on the regulation of body water and electrolytes
As oestrogen receptors in the hypothalamus had been identified in animals, we examined a role for oestrogens the in osmotic AVP regulation in humans. Specifically, we determined the slope and intercept of the P[AVP]:osmolality (POsm) and thirst:POsm linear relationships during dehydration and hypertonic saline infusion under different hormone exposures (Calzone et al. 2001; Stachenfeld et al. 2001; Stachenfeld & Keefe, 2002). In a series of studies, we demonstrated an oestrogen-associated shift to an earlier abscissal intercept or threshold for osmotic sensation of thirst and the release of AVP, with no change in the slope, or sensitivity of this relationship (Fig. 4). These shifts persisted during progestin and combined oestrogen–progestin OCP treatments, were consistent with those of earlier investigations of oestrogen effects on osmotic AVP regulation (Spruce et al. 1985; Trigoso et al. 1996), and were supported by our subsequent studies with the GnRH suppression, hormone add-back model (Fig. 5) (Stachenfeld & Keefe, 2002).
Figure 4. Mean P[AVP] and mean thirst responses to increases in plasma osmolality (POsm) during hypertonic saline infusion in the early follicular and mid-luteal phases and during oral contraceptive treatment with progesterone only and combined oestrogen and progesterone.
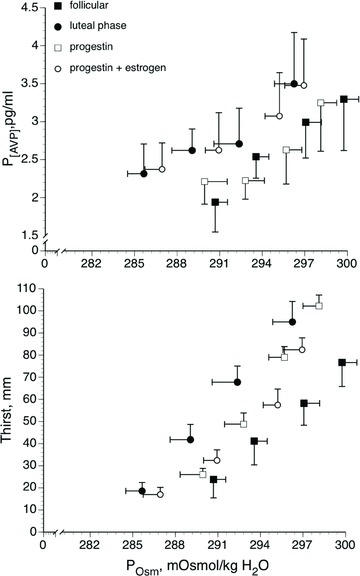
Data are mean ± SEM. From Calzone et al. (2001).
Figure 5. Mean plasma arginine vasopressin concentration (P[AVP]) responses to increases in plasma osmolality (POsm) during hypertonic saline infusion (over 105 min) during lupron administration (GnRH antagonist) alone and with 17 β-oestradiol administration.
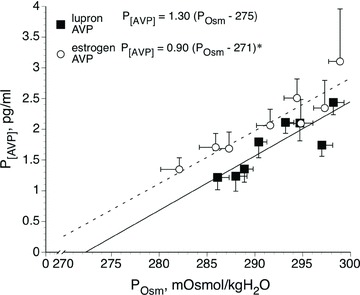
Data are mean ± SEM.*P < 0.05, GnRHa alone versus hormone treatment. From Stachenfeld & Keefe (2002).
Despite the earlier osmotic AVP release, the rate of renal free water clearance ( ) during osmotic stimulation appears unaffected by hormone exposure in women (Stachenfeld & Keefe, 2002), suggesting that these hormones may lower renal tubular sensitivity to AVP. This finding would be consistent with studies demonstrating that oestradiol attenuates the renal antidiuretic action of AVP in the rat (Carlberg et al. 1984; Wang et al. 1995). We tested this hypothesis, but our human studies examining renal concentrating response to graded synthetic AVP infusions do not support a change in renal tubular sensitivity to AVP during oestradiol administration (Fig. 6) (Stachenfeld et al. 2003). Thus, the earlier osmotic AVP release concomitant with a constant
) during osmotic stimulation appears unaffected by hormone exposure in women (Stachenfeld & Keefe, 2002), suggesting that these hormones may lower renal tubular sensitivity to AVP. This finding would be consistent with studies demonstrating that oestradiol attenuates the renal antidiuretic action of AVP in the rat (Carlberg et al. 1984; Wang et al. 1995). We tested this hypothesis, but our human studies examining renal concentrating response to graded synthetic AVP infusions do not support a change in renal tubular sensitivity to AVP during oestradiol administration (Fig. 6) (Stachenfeld et al. 2003). Thus, the earlier osmotic AVP release concomitant with a constant  during oestradiol exposure indicates a shift in the osmotic operating point for body fluid to a lower POsm.
during oestradiol exposure indicates a shift in the osmotic operating point for body fluid to a lower POsm.
Figure 6. Mean urine osmolality as a function of mean plasma arginine vasopressins concentration (P[AVP]) in response to synthetic AVP infusions duirng administration of a gonadotropin releasing agonist (luprolide, GnRH antagonist) alone and with 17 β-oestradiol.
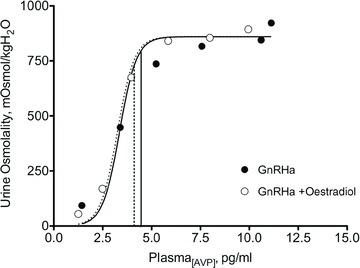
Data are mean ± SEM. (Adapted from Stachenfeld et al. (2003).
Ovarian hormone effects on hyponatraemia
One of the more important sex differences in humans is the greater risk for hyponatraemia during endurance exercise in young, healthy women compared with men of similar age. This risk has been attributed to women's lower body weight and size, excess water ingestion and longer racing times relative to men (Speedy et al. 2001; Almond et al. 2005). While these factors contribute to the greater incidence of hyponatraemia in women, oestradiol exposure also plays a role in increasing this risk (Fraser & Arieff, 1997; Ayus et al. 2000; Stachenfeld & Taylor, 2004; Stachenfeld et al. 2005). Some field studies have suggested that lower AVP response to increases in osmolality in women increases their risk for exercise-associated hyponatraemia (Siegel et al. 2007). However, our laboratory data demonstrated that osmotic regulation of AVP is not different between women with and without hyponatraemia (Stachenfeld & Taylor, 2009).
Women of reproductive age are also more likely to experience post-operative hyponatraemia (Ayus et al. 1992; Ayus & Arieff, 1993, 1996; Fraser & Arieff, 1997), especially after reproductive surgeries when oestradiol levels are increased (Amede et al. 2002). In both men and women undergoing even minor surgery, a combination of anaesthesia, post-surgical stress and nausea can lead to dramatic increases in AVP in both sexes, but greater AVP exposure is associated with brain swelling and damage primarily in women (Arieff, 1986; Ayus et al. 1992; Ayus & Arieff, 1996; Fraser & Arieff, 1997). Studies in rats have demonstrated that in response to increasing hypotonic water retention, AVP increases brain capillary and cerebroventricular ependymal cell water permeability through specific water channels (aquaporin AQP4), which are regulated via AVP-V1 receptors (Fraser et al. 1989), increasing sodium and water movement inside astrocytes. In male animals, the Na+-K+ ATPase pump acts to extrude sodium out of the astrocytes to normalize volume (Fraser & Sarnacki, 1989). However, this Na+-K+ ATPase pump action is inhibited in female rats, especially during oestradiol administration, which blocks astroglia regulatory volume decrease, resulting in greater water remaining within the cells and increasing the risk of brain damage (Fraser & Sarnacki, 1989). Thus, oestradiol may play a significant role in the greater risk of cerebral oedema and encephalopathy in women, indicating a more complex etiology than simply lower body size, and in the case of exercise-associated hyponatraemia, longer running times and cultural norms of drinking behaviour (Almond et al. 2005).
Oestrogen and progesterone effects on the regulation of body water and electrolytes in menopause
Independent of menopause, ageing has important effects on fluid balance. Although older people are generally euhydrated, ageing is associated with higher plasma osmolality and there is an age-related blunting of thirst sensation during exercise and water deprivation (Leaf, 1984; Mack et al. 1994). Most importantly, although older adults adequately restore body fluid homeostasis following dehydration or water loading, this process is slower relative to younger individuals (Phillips et al. 1984; Mack et al. 1994; Stachenfeld et al. 1996), most probably due to slower kidney function (Lindeman, 1990). Using dehydration followed by head-out water immersion (a technique that selectively restores extracellular volume while keeping osmolality high) we demonstrated that thirst sensitivity to extracellular volume change is reduced in older adults, but osmoreceptor signalling remains intact (Mack et al. 1994; Stachenfeld et al. 1997). In menopausal women oestradiol administration is associated with increases in basal P[AVP], plasma volume expansion and a downward shift in the osmotic threshold for AVP release (Stachenfeld et al. 1998). Unlike in younger women, the earlier osmotic AVP threshold is associated with greater water and sodium retention (Stachenfeld et al. 1998). Sodium also plays an important role in hypertension and progression of chronic kidney disease in postmenopausal women (Pechere-Bertschi & Burnier, 2004), and salt sensitivity correlates inversely with levels of circulating oestrogens and progesterone (Suzuki & Kondo, 2012). A recent study demonstrated greater desmopressin-induced water retention in older women, which could increase their risk of hyponatraemia (Juul et al. 2011).
Water balance during pregnancy
As with women who are not pregnant, ovarian hormones may also impact plasma volume expansion and water retention during pregnancy. Within the first few weeks of pregnancy, maternal oestrogens and progesterone exposure increase accompanied by increases in plasma and blood volume, stroke volume, heart rate and cardiac output. The last trimester of pregnancy is characterized by a rapid rise in oestrogens, which coincides with greater plasma volume and interstitial fluid expansion (Hytten, 1970). The increases in blood volume are important to support both maternal health and fetal development. This blood volume expansion is supported by a 50% increase in renal blood flow and glomerular filtration rate and greater sodium and fluid retention, mediated by the renin–angiotensin–aldosterone system and AVP (Chapman et al. 1998; Thornburg et al. 2000). Pregnancy can increase the risk for hyponatraemia (concomitant with the blood volume expansion). This hyponatraemic hypervolaemia is associated with a lower osmotic threshold for AVP release (Davison et al. 1984). This threshold shift is without a change in sensitivity (P[AVP]–POsm slope), similar to non-pregnant women during high oestrogen exposure (Stachenfeld & Keefe, 2002). Moreover, the greater AVP secretion is associated with an enhanced osmotic thirst response, perhaps leading to greater water intake as has been seen in the rat model (Brunton et al. 2008; Joyner et al. 2008).
The mechanism for these pregnancy-related changes has been investigated primarily in rats. Vasopressin and oxytocin neurons in the paraventricular (PVN) and supraoptic nuclei (SON), both located in the hypothalamus, are osmosensitive and both contribute to sodium and water regulation during pregnancy. Arginine vasopressin increases renal free water retention, while oxytocin stimulates natriuresis through an atrial natriuretic peptide (ANP) mechanism. During pregnancy, AVP osmo- and volume regulation adjusts to the blood volume expansion, shifting the osmotic threshold for AVP release and thirst to the left. As described by Brunton et al. (2008), these changes in osmoregulation of AVP are independent of nitric oxide and opiods, and a similar shift in osmoregulation of oxytocin does not occur. The exact mechanism responsible for this shift has not been determined, but both relaxin and chorionic gonadotropin (hCG) have been implicated concomitant with changes in oestrogen and progesterone exposure (Lindheimer & Davison, 1995; Brunton et al. 2008). For an excellent review on the subject see Brunton et al. (2008).
In normal pregnancy, oestrogen-related increases in nitric oxide availability and related vasodilatation reduce peripheral vascular resistance and prevent increases in blood pressure that accompany the renin–angiotensin system stimulation (Chapman et al. 1998) and blood volume expansion. Indeed, blood pressure can decrease during pregnancy in healthy women. Also during pregnancy, aortic size and compliance increase, as does venous compliance, indicating blood vessel remodelling (Thornburg et al. 2000). These haemodynamic changes occur early in pregnancy before the blood supply between the uterus and placenta is well developed.
Conclusions and perspectives
The series of studies described in this review have been central to describing the impact of ovarian hormones on the integrated systems that regulate blood pressure and body water. We have emphasized the impact of oestrogens and progesterone and have put forth the hypothesis that sex differences in these systems are primarily a function of the level of exposure to the ovarian hormones oestradiol and progesterone. Thus, physiological differences between men and women are important not only because of their obvious clinical and experimental consequences, but because of what they tell us about the physiological effects of the ovarian hormones in systems not directly involved in reproduction. Ovarian hormones are important regulators of blood pressure and water regulation systems between men and women, and they are also important with regard to these systems within women. Thus, between men and women, as well as within women, ovarian hormone exposure, and sensitivity to this exposure, contributes to blood pressure regulation, as well as disorders of autonomic function such as orthostatic intolerance and hypertension.
Glossary
- AVP
arginine vasopressin
- CO
cardiac output
- FMD
flow-mediated vasodilatation
- FSH
follicle stimulating hormone
- GnRH
gonadotropin releasing hormone
- OCP
oral contraceptive pill
- MSNA
muscle sympathetic nerve activity
- TPR
total peripheral resistance
References
- Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 2010;235:111–118. doi: 10.1258/ebm.2009.009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond CSD, Shin AY, Fortescue EB, Mannix RC, Wypij D, Binstadt BA, Duncan CN, Olson DP, Salerno AE, Newburger JW, Greenes DS. Hyponatremia among runners in the Boston Marathon. N Engl J Med. 2005;352:1550–1556. doi: 10.1056/NEJMoa043901. [DOI] [PubMed] [Google Scholar]
- Amede FJ, James KA, Michelis MF, Gleim GW. Changes in serum sodium, sodium balance, water balance, and plasma hormone levels as the result of pelvic surgery in women. Int Urol Nephrol. 2002;34:545–550. doi: 10.1023/a:1025601304345. [DOI] [PubMed] [Google Scholar]
- Arieff AI. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med. 1986;314:1529–1535. doi: 10.1056/NEJM198606123142401. [DOI] [PubMed] [Google Scholar]
- Ayus JC, Arieff AI. Pathogenesis and prevention of hyponatremic encephalopathy. Endocrinol Metab Clin North Am. 1993;22:425–446. [PubMed] [Google Scholar]
- Ayus JC, Arieff AI. Brain damage and postoperative hyponatremia: the role of gender (Review) Neurology. 1996;46:323–328. doi: 10.1212/wnl.46.2.323. [DOI] [PubMed] [Google Scholar]
- Ayus JC, Varon J, Arieff AI. Hyponatremia, cerebral edema, and noncardiogenic pulmonary edema in marathon runners. Ann Intern Med. 2000;132:711–714. doi: 10.7326/0003-4819-132-9-200005020-00005. [DOI] [PubMed] [Google Scholar]
- Ayus JC, Wheeler JM, Arieff AI. Postoperative hyponatremic encephalopathy in menstruent women. An Intern Med. 1992;117:891–897. doi: 10.7326/0003-4819-117-11-891. [DOI] [PubMed] [Google Scholar]
- Brooks VL, Cassaglia PA, Zhao D, Goldman RK. Baroreflex function in females: changes with the reproductive cycle and pregnancy. Gend Med. 2012;9:61–67. doi: 10.1016/j.genm.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks VL, Dampney RA, Heesch CM. Pregnancy and the endocrine regulation of the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol. 2010;299:R439–R451. doi: 10.1152/ajpregu.00059.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Arunachalam S, Russel JA. Control of neurohypophysial hormone secretion, blood osmolality and volume in pregnancy. J Physiol Pharmacol. 2008;59:27–45. [PubMed] [Google Scholar]
- Calzone WL, Silva C, Keefe DL, Stachenfeld NS. Progesterone does not alter osmotic regulation of AVP. Am J Physiol Regulatory Integrative Comp Physiol. 2001;281:R2011–R2020. doi: 10.1152/ajpregu.2001.281.6.R2011. [DOI] [PubMed] [Google Scholar]
- Carlberg KA, Fregley MJ, Fahey M. Effects of chronic estrogen treatment on water exchange in rats. Am J Physiol Endocrinol Metab. 1984;247:E101–E110. doi: 10.1152/ajpendo.1984.247.1.E101. [DOI] [PubMed] [Google Scholar]
- Carter JR, Klein JC, Schwartz CE. Effects of oral contraceptives on sympathetic nerve activity during orthostatic stress in young, healthy women. Am J Physiol Regul Integr Comp Physiol. 2009a;298:R9–R14. doi: 10.1152/ajpregu.00554.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab. 2009b;297:E85–E91. doi: 10.1152/ajpendo.00019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG, Dahms T, Schrier RW. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54:2056–2063. doi: 10.1046/j.1523-1755.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Barnes SA, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Relationship between muscle sympathetic nerve activity and systemic hemodynamics during nitric oxide synthase inhibition in humans. Am J Physiol Heart Circ Physiol. 2006a;291:H1378–H1383. doi: 10.1152/ajpheart.00234.2006. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol. 2006b;572:821–827. doi: 10.1113/jphysiol.2005.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111:494–498. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- Claybaugh JR, Sato AK, Crosswhite LK, Hassell LH. Effects of time of day, gender, and menstrual cycle phase on the human response to oral water load. Am J Physiol Regul Integr Comp Physiol. 2000;279:R966–R973. doi: 10.1152/ajpregu.2000.279.3.R966. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Reynolds BV, Yandl MG, Carter JR, Tahvanaainen KUO, Kuusela TA. Effects of exercise training on cardiovagal and sympthetic responses to Valsalva's maneuver. Med Sci Sports Exer. 2002;34:928–935. doi: 10.1097/00005768-200206000-00004. [DOI] [PubMed] [Google Scholar]
- Creinin MD, Lippman JS, Eder SE, Godwin AM, Olson W. The effect of extending the pill-free interval on follicular activity: triphasic norgestimate/35 μg ethinyl estradiol versus monophasic levonorgestrel/20 μg ethinyl estradiol. Contraception. 2002;66:147–152. doi: 10.1016/s0010-7824(02)00344-x. [DOI] [PubMed] [Google Scholar]
- Davison JM, Gilmore EA, Durr J, Robertson GL, Lindheimer MD. Altered osmotic thresholds for vasopressin secretion and thirst in human pregnancy. Am J Physiol Renal Physiol. 1984;246:F105–F109. doi: 10.1152/ajprenal.1984.246.1.F105. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Afify EA, Mohy El-Din MM, Omar AG, Sharabi FM. Testosterone facilitates the baroreceptor control of reflex bradycardia: role of cardiac sympathetic and parasympathetic components. J Cardiovasc Pharmacol. 2001;38:754–763. doi: 10.1097/00005344-200111000-00012. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Afify EA, Omar AG, Sharabi FM. Cyclosporine adversely affects baroreflexes via inhibition of testosterone modulation of cardiac vagal control. J Pharmacol Exp Ther. 2002;301:346–354. doi: 10.1124/jpet.301.1.346. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Meyer M, Osol G. Estrogen replacement increases beta-adrenoceptor-mediated relaxation of rat mesenteric arteries. J Vasc Res. 1996;33:124–131. doi: 10.1159/000159140. [DOI] [PubMed] [Google Scholar]
- Fraser CL, Arieff AI. Epidemiology, pathophysiology, and management of hyponatremic encephalopathy. Am J Med. 1997;102:67–77. doi: 10.1016/s0002-9343(96)00274-4. [DOI] [PubMed] [Google Scholar]
- Fraser CL, Kucharczyk J, Arieff AI, Rollin C, Sarnacki P, Norman D. Sex differences result in increased morbidity from hyponatremia in female rats. Am J Physiol Regul Integr Comp Physiol. 1989;256:R880–R885. doi: 10.1152/ajpregu.1989.256.4.R880. [DOI] [PubMed] [Google Scholar]
- Fraser CL, Sarnacki P. Na+-K+-ATPase pump function in rat brain synaptosomes is different in males and females. Am J Physiol Endocrinol Metab. 1989;257:E284–E289. doi: 10.1152/ajpendo.1989.257.2.E284. [DOI] [PubMed] [Google Scholar]
- Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol. 2009;587:2019–2031. doi: 10.1113/jphysiol.2008.168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halmos G, Sghally AV, Pinski J, Vadillo-Buenfil M, Groot K. Down-regulation of pituitary receptors for luteinizing hormone-releasing hormone (LH-RH) in rats by LH-RH antagonist Cetrorelix. Proc Natl Acad Sci U S A. 1996;93:2398–2402. doi: 10.1073/pnas.93.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the beta-adrenergic receptors. J Physiol. 2011;589:5285–5297. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol. 2012;590:2069–2079. doi: 10.1113/jphysiol.2011.224642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchhi Y. Modulation of endothelium-dependent flow mediated dilitation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92:3431–3435. doi: 10.1161/01.cir.92.12.3431. [DOI] [PubMed] [Google Scholar]
- Heesch CM, Rogers RC. Effects of pregnancy and progesterone metabolites on regulation of sympathetic outflow. Clin Exp Pharmacol & Physiol. 1995;22:136–142. doi: 10.1111/j.1440-1681.1995.tb01970.x. [DOI] [PubMed] [Google Scholar]
- Heritage AS, Stumpf WE, Sar M, Grant LD. Brainstem catecholamine neurons are target sites for sex steroid hormones. Science. 1980;207:1377–1379. doi: 10.1126/science.7355296. [DOI] [PubMed] [Google Scholar]
- Hunt BE, Taylor JA, Hamner JW, Gagnon M, Lipsitz LA. Estrogen replacement therapy improves baroreflex regulation of vascular sympathetic outflow in postmenopausal women. Circulation. 2001;103:2909–2914. doi: 10.1161/01.cir.103.24.2909. [DOI] [PubMed] [Google Scholar]
- Hvistendahl GM, Frokiaer J, Nielsen S, Djurhuus JC. Gender differences in nighttime plasma arginine vasopressin and delayed compensatory urine output in the elderly population after desmopressin. J Urol. 2007;178:2671–2676. doi: 10.1016/j.juro.2007.07.123. [DOI] [PubMed] [Google Scholar]
- Hytten FE. Water storage in normal pregnancy. Int J Gynecol Obstet. 1970;33:133–348. [Google Scholar]
- Ingegno MD, Money SR, Thelmo W, Greene GL, Davidian M, Jaffe BM, Pertschuk LP. Progesterone receptors in the human heart and great vessels. Lab Invest. 1988;59:353–356. [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus; size changes in relation to sex and age. J Clin Endocrinol Metab. 1999;84:4637–4644. doi: 10.1210/jcem.84.12.6187. [DOI] [PubMed] [Google Scholar]
- Jiang CW, Sarrel PM, Lindsay DC, Poole-Wilson PA, Collins P. Progesterone induces endothelium-independent relaxation of rabbit coronary artery in vivo. 1992;211:163–167. doi: 10.1016/0014-2999(92)90524-8. [DOI] [PubMed] [Google Scholar]
- Joyner J, Neves LAA, Stovall K, Ferrario CM, Brosnihan KB. Angiotensin-(1–7) serves as an aquaretic by increasing water intake and diuresis in association with downregulation of aquaporin-1 during pregnancy in rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1073–R1080. doi: 10.1152/ajpregu.00572.2007. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension. 2010;56:10–16. doi: 10.1161/HYPERTENSIONAHA.109.140186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul KV, Klein BM, Sandstrom R, Erichsen L, Norgaard JP. Gender difference in antidiuretic response to desmopressin. Am J Physiol Renal Physiol. 2011;300:F1116–F1122. doi: 10.1152/ajprenal.00741.2010. [DOI] [PubMed] [Google Scholar]
- Keenan N, Rosendorf K. Atlanta, GA: Centers for Disease Control and Prevention; 2011. CDC Health Disparities and Inequalities Report – United States, 2011: Morbidity and Mortality Weekly Report (MMWR) [Google Scholar]
- Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- Laiprasert JD, Rogers RC, Heesch CM. Neurosteroid modulation of arterial baroreflex-sensitive neurons in rat rostral ventrolateral medulla. Am J Physiol Reg Integrative Comp Physiol. 1998;247:R903–R911. doi: 10.1152/ajpregu.1998.274.4.R903. [DOI] [PubMed] [Google Scholar]
- Leaf A. Dehydration in the elderly. N Engl J Med. 1984;311:791–792. doi: 10.1056/NEJM198409203111209. [DOI] [PubMed] [Google Scholar]
- Lee WS, Harder JA, Yoshizumi M, Lee ME. Progesterone inhibits arterial smooth muscle cell proliferation. Nat Med. 1997;3:1005–1008. doi: 10.1038/nm0997-1005. [DOI] [PubMed] [Google Scholar]
- Levenson J, Pessana F, Gariepy J, Armentano R, Simon A. Gender differences in wall shear-mediated brachial artery vasoconstriction and vasodilation. J Am Coll Cardiol. 2001;38:1668–1674. doi: 10.1016/s0735-1097(01)01604-7. [DOI] [PubMed] [Google Scholar]
- Lin AL, McGill HCJ, Sain SA. Hormone receptors of the baboon cardiovascular system. Biochemical characterization of aortic and myocardioal cytoplasmic progesterone receptors. Circ Res. 1982;50:610–616. doi: 10.1161/01.res.50.5.610. [DOI] [PubMed] [Google Scholar]
- Lindeman RD. Overview: Renal physiology and pathophysiology of aging. Am J Kid Dis. 1990;4:275–282. doi: 10.1016/s0272-6386(12)80002-3. [DOI] [PubMed] [Google Scholar]
- Lindheimer MD, Davison JM. Osmoregulation, the secretion of arginine vasopressin and its metabolism during pregnancy. Eur J Endocrinol. 1995;132:133–143. doi: 10.1530/eje.0.1320133. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Mack GW, Weseman CA, Langhans GW, Scherzer H, Gillen CM, Nadel ER. Body fluid balance in dehydrated healthy older men: thirst and renal osmoregulation. J Appl Physiol. 1994;76:1615–1623. doi: 10.1152/jappl.1994.76.4.1615. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Sugiyama Y, Watanabe T, Kobayashi F, Mano T. Gender difference in age-related changes in muscle sympathetic nerve activity in healthy subjects. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1600–R1604. doi: 10.1152/ajpregu.1998.275.5.R1600. [DOI] [PubMed] [Google Scholar]
- Meendering JR, Torgrimson BN, Miller NP, Kaplan PF, Minson CT. Estrogen, medroxyprogesterone acetate, endothelial function, and biomarkers of cardiovascular risk in young women. Am J Physiol Heart Circ Physiol. 2008;294:H1630–H1637. doi: 10.1152/ajpheart.01314.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JA, Martini ER, Smith MM, Brunt VE, Kaplan PF, Halliwill JR, Minson CT. Short-term oral progesterone administration antagonizes the effect of transdermal estradiol on endothelium-dependent vasodilation in young healthy women. Am J Physiol Heart Circ Physiol. 2011;301:H1716–H1722. doi: 10.1152/ajpheart.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young T, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000a;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation. 2000b;102:1473–1476. doi: 10.1161/01.cir.102.13.1473. [DOI] [PubMed] [Google Scholar]
- Morey AK, Pedram A, Razandi MN, Prins BA, Hu RM, Biesada E, Levin ER. Estrogen and progesterone inhibit vascular smooth muscle proliferation. Endocrinol. 1997;138:3330–3339. doi: 10.1210/endo.138.8.5354. [DOI] [PubMed] [Google Scholar]
- Mosher W, Jones J. 2010. Use of Contraception in the United States: 1982–2008, ed. Stat NCfHSVH. Centers for Disease Control and Prevention. PubMed (in Vital Health Stat 23, 1–44)
- Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- Oberye JJL, Mannaerts BMJL, Huisman JAM, Timmer CJ. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part I. Absolute bioavailability of 0.25 mg of ganirelix after a single subcutaneous injection in healthy female volunteers. Fertil Steril. 1999a;72:1001–1005. doi: 10.1016/s0015-0282(99)00413-6. [DOI] [PubMed] [Google Scholar]
- Oberye JJL, Mannaerts BMJL, Huisman JAM, Timmer CJ. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part II. Dose-proportionality and gonadotropin suppression after multiple doses of ganirelix in healthy female volunteers. Fertil Steril. 1999b;72:1006–1012. doi: 10.1016/s0015-0282(99)00414-8. [DOI] [PubMed] [Google Scholar]
- Olivennes F. The use of gonadotropin-releasing hormone antagonist in ovarian stimulation. Clin Obstet Gynecol. 2006;49:12–22. doi: 10.1097/01.grf.0000197520.53682.32. [DOI] [PubMed] [Google Scholar]
- Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- Pechere-Bertschi A, Burnier M. Female sex hormones, salt, and blood pressure regulation[ast] Am J Hypertens. 2004;17:994–1001. doi: 10.1016/j.amjhyper.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Perusquia M, Hernandez R, Morales MA, Campos MG, Villalon CM. Role of endothelium in the vasodilating effect of progestins and androgens on the rat thoracic aorta. Gen Pharmacol. 1996;27:181–185. doi: 10.1016/0306-3623(95)00091-7. [DOI] [PubMed] [Google Scholar]
- Phillips PA, Rolls BJ, Ledingham JGG. Body fluid changes, thirst and drinking in man during free access to water. Physiol Behav. 1984;33:357–363. doi: 10.1016/0031-9384(84)90154-9. [DOI] [PubMed] [Google Scholar]
- Reckelhoff JF, Granger JP. Role of androgens in mediating hypertension and renal injury. Clin Experimental Pharmacol Physiol. 1999;26:127–131. doi: 10.1046/j.1440-1681.1999.02996.x. [DOI] [PubMed] [Google Scholar]
- Roesch DM, Keller-Wood M. Progesterone rapidly reduces arterial blood pressure in ewes. Am J Physiol Heart Circ Physiol. 1997;272:H386–H391. doi: 10.1152/ajpheart.1997.272.1.H386. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sar M, Stumpf WE. Simultaneous localization of [3H]estradiol and neurophysin I or arginine vasopressin in hypothalamic neurons demonstrated by a combined technique of dry-mount autoradiography and immunohistochemistry. Neurosci Lett. 1980;17:179–184. doi: 10.1016/0304-3940(80)90081-6. [DOI] [PubMed] [Google Scholar]
- Schlaff WD, Lynch AM, Hughes HD, Cedars MI, Smith DL. Manipulation of the pill-free interval in oral conceptive pill users: the effect on follicular suppression. Am J Obstet Gynecol. 2004;190:943–951. doi: 10.1016/j.ajog.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Joyner MJ, Charkoudian N, Wallin BG, Hart EC. Sex differences in alpha-adrenergic support of blood pressure. Clin Auton Res. 2010;20:271–275. doi: 10.1007/s10286-010-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel AJ, Verbalis JG, Clement S, Mendelson JH, Mello NK, Adner M, Shirey T, Glowacki J, Lee-Lewandrowski E, Lewandrowski KB. Hyponatremia in marathon runners due to inappropriate arginine vasopressin secretion. Am J Med. 2007;120:461. doi: 10.1016/j.amjmed.2006.10.027. e411–e467. [DOI] [PubMed] [Google Scholar]
- Sokolnicki LA, Khosla S, Charkoudian N. Effects of testosterone and estradiol on cutaneous vasodilation during local warming in older men. Am J Physiol Endocrinol Metab. 2007;293:E1426–E1429. doi: 10.1152/ajpendo.00535.2007. [DOI] [PubMed] [Google Scholar]
- Speedy DB, Noakes TD, Schneider C. Exercise-associated hyponatremia: A review. Emergency Med. 2001;13:17–27. doi: 10.1046/j.1442-2026.2001.00173.x. [DOI] [PubMed] [Google Scholar]
- Speroff L, Glass RH, Kase NG. Clinical Gynecological Endocrinology and Infertility. Baltimore: Williams & Wilkins; 1999. Steroid contraception; pp. 873–879. [Google Scholar]
- Spruce BA, Baylis PH, Burd J, Watson MJ. Variation in osmoregulation of arginine vasopressin during the human menstrual cycle. Clin Endocrinol. 1985;22:37–42. doi: 10.1111/j.1365-2265.1985.tb01062.x. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, DiPietro L, Palter SF, Nadel ER. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am J Physiol Regul Integr Comp Physiol. 1998;274:R187–R195. doi: 10.1152/ajpregu.1998.274.1.R187. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Keefe DL. Estrogen effects on osmotic regulation of AVP and fluid balance. Am J Physiol Endocrinol Metab. 2002;283:E711–E721. doi: 10.1152/ajpendo.00192.2002. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Keefe DL, Taylor HS. Responses to a saline load in GnRH antagonist-pretreated premenopausal women on progesterone or estradiol-progesterone therapy. J Clin Endocrinol Metab. 2005;90:386–394. doi: 10.1210/jc.2004-0941. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Mack GW, DiPietro L, Nadel ER. Mechanism for attenuated thirst in aging: role of central volume receptors. Am J Physiol Regul Integr Comp Physiol. 1997;272:R148–R157. doi: 10.1152/ajpregu.1997.272.1.R148. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Mack GW, Takamata A, DiPietro L, Nadel ER. Thirst and fluid regulatory responses to hypertonicity in older adults. Am J Physiol Regul Integr Comp Physiol. 1996;271:R757–R765. doi: 10.1152/ajpregu.1996.271.3.R757. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Splenser AE, Calzone WL, Taylor MP, Keefe DL. Sex differences in osmotic regulation of AVP and renal sodium handling. J Appl Physiol. 2001;91:1893–1901. doi: 10.1152/jappl.2001.91.4.1893. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Taylor HS. Sex hormone effects on body fluid and sodium regulation in women with and without exercise-associated hyponatremia. J Appl Physiol. 2009;107:864–872. doi: 10.1152/japplphysiol.91211.2008. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Taylor HS, Leone CA, Keefe DL. Oestrogen effects on urine concentrating response in young women. J Physiol. 2003;552:869–880. doi: 10.1113/jphysiol.2003.046920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachenfeld NS, Taylor HS. Effects of estrogen and progesterone administration on extracellular fluid. J Appl Physiol. 2004;96:1011–1018. doi: 10.1152/japplphysiol.01032.2003. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Elser MD, Jennings GL, Komesaroff PA. Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension. 1997;30:1538–1543. doi: 10.1161/01.hyp.30.6.1538. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kondo K. Chronic kidney disease in postmenopausal women. Hypertens Res. 2012;35:142–147. doi: 10.1038/hr.2011.155. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Sato M, Umehara S, Nishikawa T. Influence of menstrual cycle on baroreflex control of heart rate: comparison with male volunteers. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1091–R1097. doi: 10.1152/ajpregu.00162.2003. [DOI] [PubMed] [Google Scholar]
- Tank J, Diedrich A, Szczech E, Luft FC, Jordan J. Baroreflex regulation of heart rate and sympathetic vasomotor tone in women and men. Hypertension. 2005;45:1159–1164. doi: 10.1161/01.HYP.0000165695.98915.9a. [DOI] [PubMed] [Google Scholar]
- Taylor JE, Miller BT, Gray KD, Scott RT, Jr, Catherino WH, Segars JH. The mechanism responsible for the supraphysiologic gonadotropin surge in females treated with gonadotropin-releasing hormone (GnRH) agonist and primed with GnRH antagonist. Fertil Steril. 2010;93:1668–1675. doi: 10.1016/j.fertnstert.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Semin Perinatol. 2000;24:11–14. doi: 10.1016/s0146-0005(00)80047-6. [DOI] [PubMed] [Google Scholar]
- Trigoso WF, Wesly JM, Meranda DL, Shenker Y. Vasopressin and atrial natriuretic peptide hormone responses to hypertonic saline infusion during the follicular and luteal phases of the menstrual cycle. Hum Reprod. 1996;11:2392–2395. doi: 10.1093/oxfordjournals.humrep.a019121. [DOI] [PubMed] [Google Scholar]
- van Heusden AM, Fauser BCJM. Activity of the pituitary-ovarian axis in the pill-free interval during use of low-dose combined oral contraceptives. Contraception. 1999;59:237–243. doi: 10.1016/s0010-7824(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Vongpatanasin W, Tuncel M, Mansour Y, Arbique D, Victor RG. Transdermal estrogen replacement therapy decreases sympathetic activity in postmenopausal women. Circulation. 2001;103:2903–2908. doi: 10.1161/01.cir.103.24.2903. [DOI] [PubMed] [Google Scholar]
- Wang Y, Crofton JT, Liu H, Sato K, Brooks DP, Share L. Estradiol attenuates the antidiuretic action of vasopressin in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 1995;268:R951–R957. doi: 10.1152/ajpregu.1995.268.4.R951. [DOI] [PubMed] [Google Scholar]
- Wenner MM, Taylor HS, Stachenfeld N. Endothelin B receptor contribution to peripheral microvascular function in women with polycystic ovary syndrome. J Physiol. 2011a;589:4671–4679. doi: 10.1113/jphysiol.2011.216218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner MM, Taylor HS, Stachenfeld NS. Progesterone enhances adrenergic control of skin blood flow in women with high but not low orthostatic tolerance. J Physiol. 2011b;589:975–986. doi: 10.1113/jphysiol.2010.194563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2012. What do we mean by “sex” and “gender”? http://www.who.int/gender/whatisgender/en/
- Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab. 2001;86:5389–5395. doi: 10.1210/jcem.86.11.8013. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Davidge ST. Effect of estrogen replacement on vasoconstrictor responses in rat mesenteric arteries. Hypertension. 1999;34:1117–1122. doi: 10.1161/01.hyp.34.5.1117. [DOI] [PubMed] [Google Scholar]


