Figure 1. Overview of extra- and intracellular processing of SCFA-hexosamine analogs.
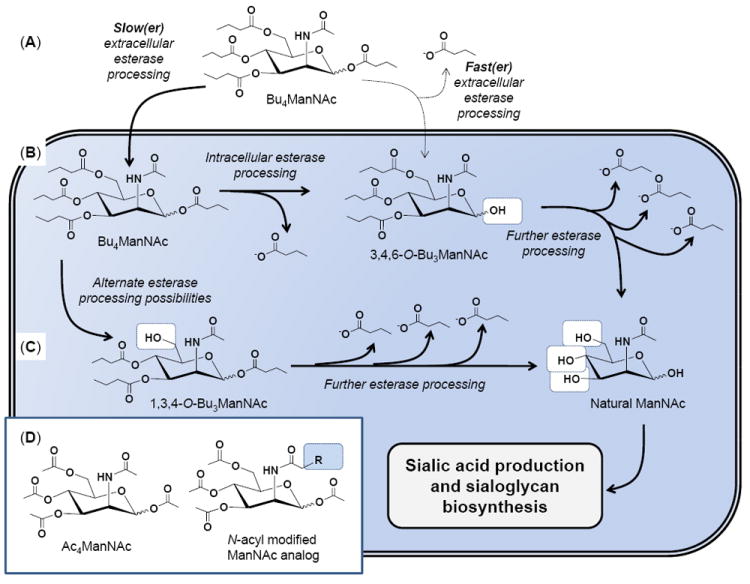
(A) The lead compound Bu4ManNAc is used for illustrative purposes; if extracellular esterase/lipase degradation is relatively slow (left) then the intact molecule will enter the cell; alternately if ester hydrolysis is relatively fast (right) a partially degraded metabolite such as 3,4,6-O-Bu3ManNAc will enter the cell (B). In either case, the butanoylated analog is subject to further esterase processing to ultimately produce fully deprotected ManNAc (B), which can also be derived from 1,3,4-O-Bu3ManNAc (or other partially deprotected intermediates, not shown) (C) and used by the sialic acid biosynthetic pathway (as described in detail elsewhere14,35) to produced sialic acid and sialylated glycans. (D) Similar esterase processing occurs for peracetylated ManNAc (illustrated) and its hydrolysis products as well as to N-acyl, R-modified analogs (such as the azido analogs shown in Fig. 2D).
