Figure 3. Intracellular esterase processing and an explanation for slow extracellular and fast intracellular analog hydrolysis.
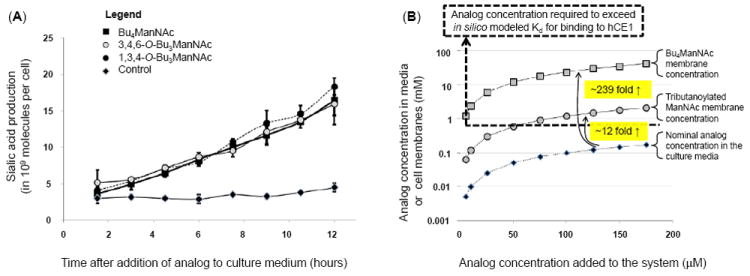
(A) Sialic acid production in LS174T “indicator cells” incubated with different forms of butanoylated ManNAc analogs shows statistical no difference over the first several hours. (B) Theoretical explanation for the slow extracellular and rapid intracellular processing of SCFA-ManNAc analogs: tributanoylated and Bu4ManNAc concentrations are estimated in solution and in (or near) membranes based on logP values calculated with ChemDraw. The Kd for association with hCE1 (a plausible model esterase) indicated with the dashed line, showing that the nominal concentration for analog in solution in media is typically below this threshold resulting in slow extracellular hydrolysis of SCFA groups while membrane-associated analog is typically at concentrations higher than this threshold, thus explaining the rapid intracellular hydrolysis of SCFA groups.
