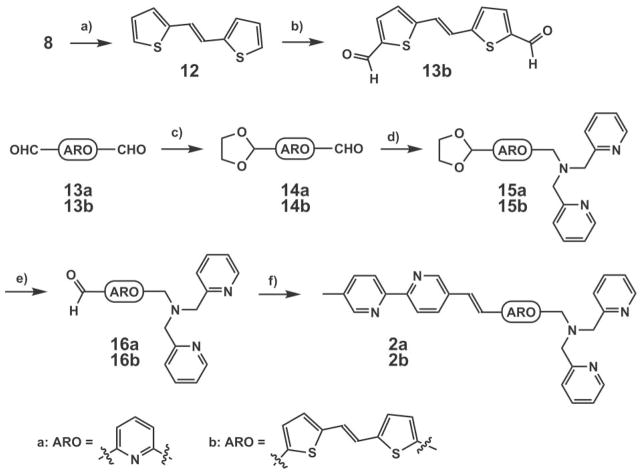
Scheme 2.
Syntheses of 2a and 2b. a) NaH, DME, 2-thiophene-carboxaldehyde, 88%; b) POCl3, DMF, reflux, 98%; c) ethylene glycol, TsOH (catalyst), benzene, Dean–Stark, reflux, 20% for 14a; d) di(2-picolyl)amine, NaBH(OAc)3, rt, 58% for 15a; e) HCl–THF–H2 O, rt; f) NaH, dimethoxyethane, 5, rt, 46% for 2a and 12% for 2b in two steps.