Abstract
The objective of the study was to evaluate the hepatoprotective activity of Gumma (Leucas cephalotes Spreng.) against carbon tetrachloride (CCl4) induced hepatotoxicity. Hepatic damage was induced by the administration of CCl4 in wistar rats. Hydro alcoholic (1:1) extract of Gumma (583mg/kg orally) was used to screen the extent of hepatoprotection in the rats subjected to hepatotoxicity. The protective effect was compared against the plain control and negative control groups treated with distilled water (p.o.) and CCl4 in the dose of 0.7ml/kg, i.p. daily. The liver function test and histopathological examination of liver tissue was conducted to assess the hepatoprotective effect. The comparison among different treatments was carried out using one way ANOVA with post hoc Dunnett's multiple comparison test. The mean serum markers of liver function in plain control for Bilirubin, AST, ALT and ALKP were 0.69 ± 0.04, 34.85 ± 4.45, 16.84 ± 4.64 and 33.85 ± 3.20 whereas in CCl4 treated group, the values were found to be 4.42 ± 0.71, 99.33 ± 13.89, 127.83 ± 6.55 and 70.83 ± 11.26, respectively. In pre-treated group, Bilirubin, AST, ALT and ALKP were observed to be 0.67 ± 0.03, 32.97 ± 7.77, 42.57 ± 2.03 and 42.42 ± 4.41 while in post-treated 6.68 ± 0.90, 145.66 ± 33.37, 44.16 ± 5.16 and 56.83 ± 10.44, and in concurrent group 0.24 ± 0.07, 21 ± 3.66, 37.48 ± 4.38 and 40.4 ± 7.03, respectively.
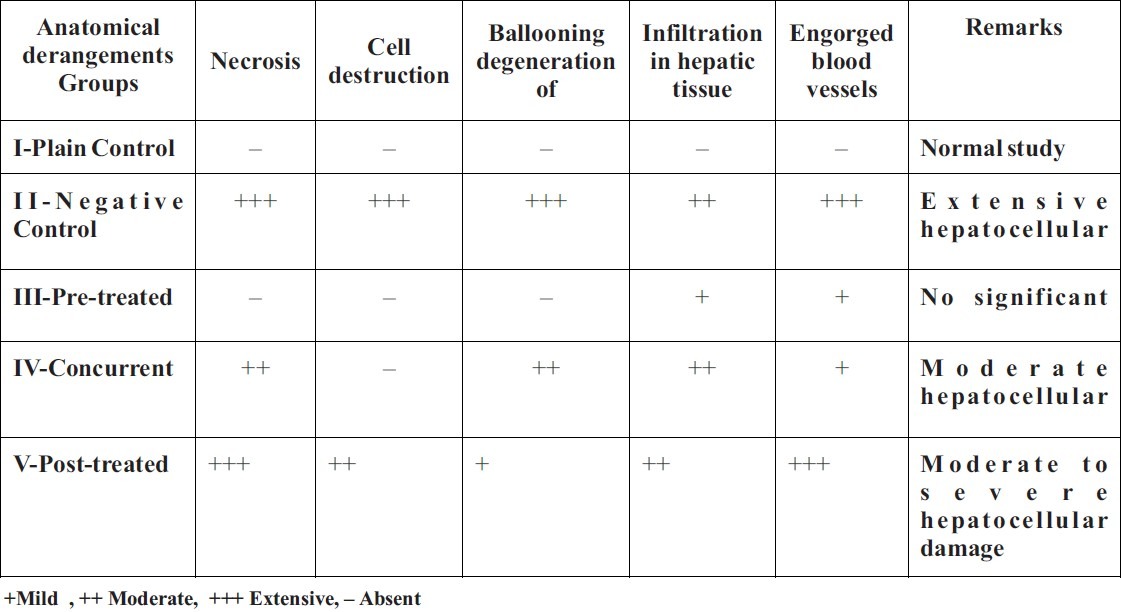
Histologically, no significant pathology was observed in the liver of Plain control group, while extensive hepatocellular damage was seen in CCl4 treated group. Pre-treated group showed no significant damage, while liver of Concurrent group showed moderate hepatocellular damage, whereas Post-treated group showed moderate to severe hepatocellular damage. The extract of Gumma (Leucas cephalotes Spreng.) caused significant lowering of serum markers of liver function to near normal in chemically challenged animals (p<0.01). It also reversed the liver tissue damage caused by CCl4. Prophylactic effect has been more marked than the curative effect. Thus, the findings suggest that Gumma possesses hepatoprotective effect.
Keywords: Hepatotoxicity, Carbon tetrachloride, Hydroalcoholic extract, Liver function
Introduction
An important drug Leucas cephalotes Spreng called as Gumma[1] in Unani medicine was selected for the present study on the basis of its ascribed effect as hepatoprotective, and the age-old practice of Unani physicians, particularly in India to prescribe it in the management of liver diseases. Gumma (Leucas cephalotes Spreng.) belongs to the family Lamiaceae.It is an erect, annual hairy and pubescent herb, 30-100 cm high, found as a common weed in cultivated grounds especially after a period of rain and in waste lands throughout the greater part of India, ascending upto 1,800 meter in Himalayas[2,3]. The whole plant increases appetite if used as food4]. The whole plant extract in an oral dose of 20 ml twice a day in jaundice is claimed to be effective by local people of villages in U.P., M.P. and Bihar states of India[5]. It has been described in ethnobotanical literature to be effective in Jaundice[6,7,8], Phlegmatic humour,Cough[2], Gastric complaints[8], Snake Bite[1], Dysentery[9] etc.
The present study was designed to investigate the hepatoprotective effect of Gumma in experimental animals by carbon tetrachloride induced hepatotoxicity. CCl4 induced hepatotoxicity in experimental animals is considered standard model to evaluate the hepatoprotective effect of test drug[10] and pathology developed by CCl4 has been considered to be very much similar to the hepatitis in human beings. Earlier study on Gumma (LCS) by Singh et al[11] showed that the ethyl acetate extract of Leucas cephalotes (whole plant) failed to protect CCl4 induced hepatotoxicity in mice and rats when used in a dose of 300 mg/kg. The present study was carried on 50% ethanolic extract at the dose of 583mg/kg, as previous study has shown it to be safe upto 1680 ± 21 mg/kg[11].
Material and Methods
Animal
The healthy adult albino rats of Wistar strain of sex, weighing 150-300gm, 2-3 months old were used in the study. They were procured from the animal house, National Institute of Unani Medicine, Bangalore. The rats were housed in polypropylene cages, under controlled conditions of light and temperature. They were fed with standard commercial food pellets (AMRUT Rat & Mice feeds, Pranav Agro Industries Ltd. Sangli) and water ad libitum. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC), National Institute of Unani Medicine, Bangalore, Karnataka, India (Reg.No. 953/C/06/CPCSEA) dated 07/12/2007.
Plant material
The plants of Gumma (Leucas cephalotes Spreng.) in full bloom were collected from the areas of Nanpara, Gonda district of Uttar Pradesh, India, during September–October 2007. The botanical identification was confirmed by the expert committee (consisting of pharmacognocist, chemist and Unani expert) of National Institute of Unani Medicine, Bangalore and a voucher specimen (No. yasir/05) has been kept in the laboratory for future reference.
Materials for Experiment
Carbon tetrachloride was obtained from the NICE Chemical Pvt. Ltd. Cochin, Kerala (India). LFT kit for the estimation of biochemical parameters was obtained from BAYER Diagnostics India Ltd. Baroda, Gujarat (India). Ethyl Alcohol (99.9%); Analytical reagent for making hydro-alcoholic extract of the test drug, was obtained from Changshu Yangyuan Chemical China.
Preparation of Extract: The whole plant of Gumma (Leucas cephalotes Spreng.) was dried in the shade and was powdered mechanically. The powder thus obtained, was extracted in Hydro alcoholic solution in the ratio of 1:1, as described below:
100 gm of powdered drug was taken into 500 ml of hydro alcoholic solution and subjected to extraction by Soxhlet apparatus. The heat was applied for 8 hrs. The extract was cooled, filtered and concentrated on water bath. The extract was weighed and yield percentage was calculated with reference to the weight of the crude drug. The yield was found to be 11% w/w.
Dosage of drug: The extract was suspended in distilled water just before the oral administration. The dosage of the drug extract for animals was extrapolated from the human therapeutic dose as described by Unani physicians. The crude dosage was multiplied by factor 7 for rats[12]. The dosage thus calculated for Gumma was found to be 583mg/kg body weight. The dosage of carbon tetrachloride was taken from standard reference[13].
Hepatoprotective Study
The study was conducted as described by Yogesh Dwiwedi et.al.[14]. A total of 35 rats were equally divided into 5 groups (n =7). Group-I which served as plain control, was given distilled water (2ml/kg/day orally) for 7 days. Liver damage was induced in rats of Group-II (Negative control), with CCl4 in the dose of 0.7ml/kg i.p. per day for 7 days. Group-III was pre-treated with hydro alcoholic extract of Gumma (583mg/kg/day orally) only for first 7 days and along with CCl4 (0.7ml/kg/day i.p) for next 7 days. Thereafter, Group-IV was given hydro alcoholic extract of Gumma (583mg/kg/day orally) and CCl4 (0.7ml/kg/day i.p) simultaneously for 7 days. Group-V was given CCl4 (0.7ml/kg/day i.p) for first 7 days and hydro alcoholic extract of Gumma (583mg/kg/day orally) along with CCl4 for next 7 days.
Collection of blood sample & liver tissue: After 7 days of treatment in the Group-I, II & IV and after 14 days treatment in the Groups-III & V, the rats were anaesthetized and the blood was collected from carotid artery for assay of serum to determine the marker enzymes of liver function viz. AST, ALT, ALKP and S. bilirubin[15]. Liver of two rats from each group was dissected out randomly after the animals were sacrificed, and was fixed in 10% formaline. Transverse section of the liver was cut and stained with eosin and hematoxylin for observing the histopathological changes.
The observations in various groups were expressed as mean ± SEM. The liver function parameters of various groups were compared with the parameters of plain and negative control groups. The inter group comparison was analyzed using statistical test of ANOVA non repeated measure with post hoc Dunnett's multiple pair comparison test. The difference in means was regarded significant at p<0.05.
Results
The mean Serum Bilirubin estimated from Group-I was found to be 0.69 ± 0.04, Group-II 4.42 ± 0.71, Group-III 0.67 ± 0.03, Group-IV 0.24 ± 0.07, and Group-V 6.68 ± 0.90. When means of serum bilirubin of various groups were compared with Group-I, it was significantly increased (p<0.01) in Group-II and Group-V, while other groups were not found significant. When means of serum bilirubin of various groups were compared with Group-II, it was found to be increased in Group-V (p<0.05) while decreased in Group-I, Group-III and Group-IV (p<0.01).
The mean Aspartate aminotransferase (AST/SGOT) in Group-I was found to be 34.85 ± 4.45, Group-II 99.33 ± 13.89, Group-III 32.97 ± 7.77, Group-IV 21 ± 3.66 and Group-V 145.66 ± 33.37. When means of AST of various groups were compared with Group-I, it was significantly increased (p<0.01) in Group-II and Group-V, while other groups were not significant. When mean AST of various groups were compared with Group-II, it was found to be decreased in Group-I, Group-III, Group-IV (p<0.01), while Group-V was not significantly different (p>0.05).
The mean of Alanine aminotransferase (ALT/SGPT) estimated for Group-I was found to be 16.84 ± 4.64, Group-II 127.83 ± 6.55, Group-III 42.57 ± 2.03, Group-IV 37.48 ± 4.38, and Group-V 44.16 ± 5.16. The mean ALT of various groups were compared with Group-I, and it was observed that mean ALT of Group-II was highly significantly increased (p<0.01) and Group-III and Group-V were significantly increased (p<0.05) while Group-IV was not significant. When mean of ALT of various groups were compared with Group-II, it was found to be decreased in all the groups (p<0.01) except Group-I (p>0.05).
The mean of Alkaline phosphatase (ALKP) estimated for Group-I was found to be 33.85 ± 3.20, Group-II 70.83 ± 11.26, Group-III 42.42 ± 4.41, Group-IV 40.4 ± 7.03 and Group-V 56.83 ± 10.44. When mean of ALKP of various groups were compared with Group-I, it was significantly increased (p<0.01) in Group-II, while other groups were not significant (p>0.05). When means of ALKP of various groups were compared with Group-II, it was found to be decreased in Group-I (p<0.01) while other remaining groups were not significantly different (p>0.05).
The results with statistical summary are shown in the Table-1. The morphological changes seen in the liver section of various groups are summarized in Table-2 and photographs of the histopathological studies are attached in the list of slides.
Table 1.
Effect of hydro alcoholic extract of Gumma (LCS) on serum marker of liver function in CCl4 induced hepatotoxicity rat model
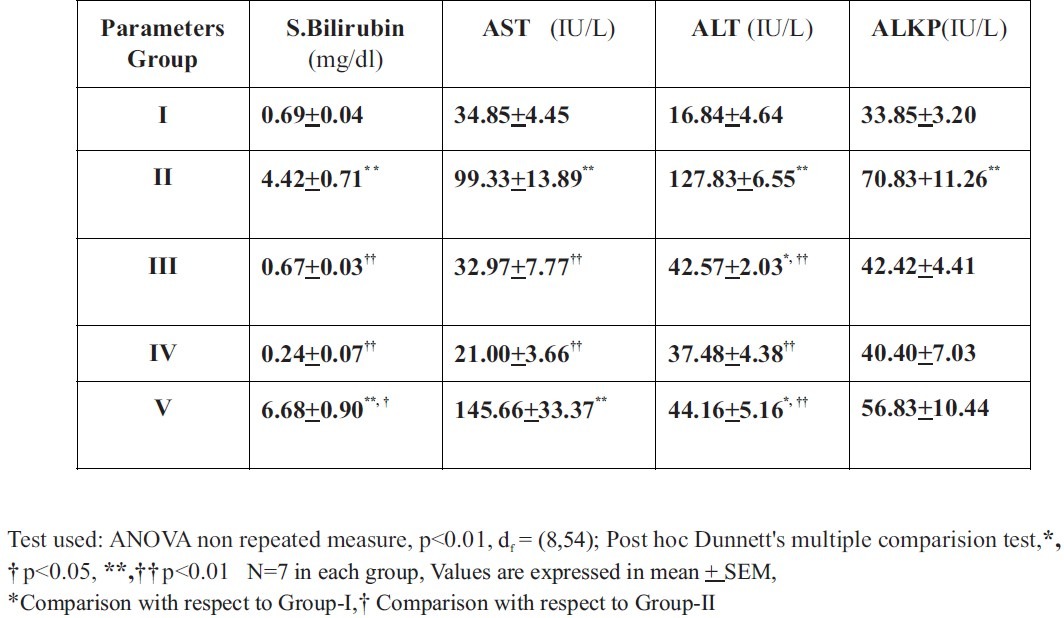
Table No.2-.
Effect of Gumma (LCS) on histopathological changes in cut section of the liver
Discussion
The study demonstrated that Gumma (LCS) possesses significant hepatoprotective activity against CCl4 induced hepatotoxicity, in the dose of 583mg/kg. This finding is not in consonance with the previous finding that did not demonstrate hepatoprotective effect in Gumma. This may be due to the fact that previous workers used relatively low doses. The hepatotoxic potential of CCl4 was reliably established with the dose of 0.7ml/kg in the present study, as evidenced by significant (p<0.01) elevations in the serum bilirubin, AST, ALT and ALKP in the groups of animals treated with CCl4. Hepatotoxic effects were further corroborated by the histological findings, which showed extensive hepatocellular damage (Slide 2). While in plain control group, the serum bilirubin, AST, ALT and ALKP levels were found within normal limit and in histopathological studies, no pathological changes were observed (Slide 1). The comparison of two groups, however demonstrated significant difference in the findings, suggesting severe hepatotoxicity induced by CCl4 as compared to the plain control group.
Slide-2.
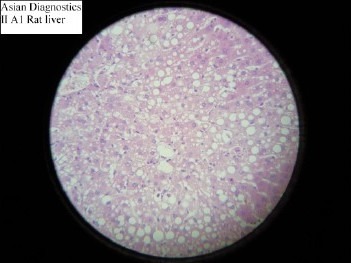
Histopathological study of Negative control
Slide-1.
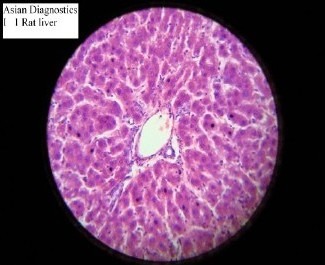
Histopathological study of Plain control group
In pre-treated group, in which animals were treated with Gumma (LCS) prior to receiving CCl4, the serum bilirubin, AST, ALT and ALKP were found to be significantly decreased when compared with the negative control group (p<0.01). Histopathological slides did not demonstrate any significant hepatocellular damage. Almost similar features were observed in control group suggesting that the structural integrity of hepatic cells was maintained (Slide 3).
Slide-3.
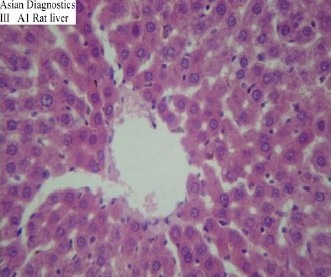
Histopathological study of Pre-treated Group
The animals in post-treated group, treated initially with CCl4 and subsequently with the test drug i.e. Gumma (LCS), demonstrated a significant decrease in ALT and ALKP level but not in S. bilirubin and AST level as compared to the negative control group. Histopathological studies too demonstrated moderate to severe hepatocellular damage (Slide 5). Thus, the two groups, pre-treated and post-treated showed that the test drug produced significant degree of hepatoprotection as all the biochemical markers of liver function and the histopathological features clearly demonstrated significant decrease in the findings of these two groups, as compared to the negative control group. Inter group comparison of pre-treated and post-treated groups however showed that the effect in pre-treated group was more significant; indicating that the protective effect of Gumma (LCS) is more striking than its curative effect.
Slide-5.
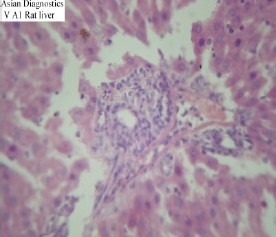
Histopathological study of Post treated group
Fig. Histological changes shown in different groups
In Concurrent test group, the Serum bilirubin, AST, ALT and ALKP were found to be significantly decreased when compared with the negative control group (p<0.01). Histological feature showed moderate hepatocellular damage (Slide 4). The effect was significant but the degree was lesser than the pre-treated group.
Slide-4.
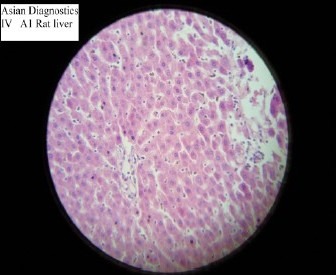
Histopathological study of Concurrent Group
The severity of liver cell damage is assessed by serial measurement of serum total bilirubin, albumin, and transaminase. The diagnosis of minimal hepatocellular damage may be suspected by observing minimally elevated serum transaminase values and sometimes serum bilirubin. Liver converts bilirubin to a polar form that can be excreted in bile and to some extent in urine. Failure to metabolize bilirubin results in jaundice[16]. The level of alkaline phosphatase rises in cholestasis and to a lesser extent when liver cells are damaged. Aspartate transaminase is a mitochondrial enzyme present in large quantity in heart, liver, skeletal muscle and kidney and the serum level increases whenever these tissues are acutely destroyed, presumably due to their release from damaged cells. Alanine transaminase is a cytosolic enzyme also present in liver. Although the absolute amount is less than AST, a greater proportion is present in liver compared with heart and skeletal muscles[17].
Since the test drug mediated reduction in levels of AST and ALT towards the respective normal values in group of animals subjected to hepatotoxicity with CCl4, the findings of the present study therefore, can be taken as an indication of stabilization of plasma membrane as well as repair of hepatic tissue damage caused by CCl4. This effect is in consonance with the commonly accepted view that serum level of transaminase return to normal with the healing of hepatic parenchyma and regeneration of hepatocytes. Alkaline phosphatase (ALKP) is the prototype of these enzymes that reflect the pathological alteration in biliary flow[18]. The test drug mediated suppression of the increased serum ALKP activity suggests the possibility of the test drug being able to stabilize biliary dysfunction in rat liver during hepatic injury with CCl4. Among the various mechanisms involved in the hepatotoxic effect of CCl4, one is oxidative damage through free radical generation, thus antioxidation property may be the likely mechanism of hepatoprotective effect of Gumma.
Earlier study on Gumma by Singh et al.[11] showed that the ethyl acetate extract of Leucas cephalotes (whole plant) failed to protect CCl4 induced hepatotoxicity in mice and rats when used in a dose of 300 mg/kg, which was confirmed histologically. The extract also failed to reduce the CCl4 induced mortality in 48 hr. The LD50 of this extract in mice was 1680±21 mg/kg i.p. Further the study of Sharma et al.[19] showed that the 90% ethanolic extract of the aerial parts of Leucas cephalotes was devoid of such an activity. The present study was carried on 50% ethanolic extract at the dose of 583mg/kg, which was higher than that used by Singh et al. Further, ethyl acetate extract may have not possessed sufficient active chemical ingredients as water soluble ingredients might have been left out. Use of hydro alcoholic extract is supported by literature indicating that it is a better solution for study the herbal drugs[20]. Further studies are needed to evaluate various chemical constituents of this drug so as to correlate its effect with those particular constituents.
Conclusion
It can be conclude that Gumma (LCS) possesses significant degree of hepatoprotective effect against carbon tetrachloride induced hepatotoxicity and if Gumma extract used as a prophylactic measure will serve as better hepatoprotective agent and ameliorate various liver diseases particularly of acute nature. The drug probably produced effect by inhibition of oxidative stress, because carbontetrachloride causes hepatic damage via oxidative degeneration, confirmation of which needs further studies.
References
- 1.Medicinal Plants in Folklores of Northern India. 1st edition. New Delhi: CCRUM, Ministry of H & F W. Govt. of India; 2001. Anonymous; p. 320. [Google Scholar]
- 2.The Wealth of India. VI. New Delhi: Council of Scientific and Industrial Research; 2003. Anonymous; pp. 79–80. [Google Scholar]
- 3.Yukinori M, Akiko S, Tsuyoshi T. Studies on Nepalese Crude Drugs. XXIX. Chemical Constituents of Dronapuspi, the Whole Herb of Leucas cephalotes Spreng. Chemical & Pharmaceutical Bulletin. 2006;54(10):1370–1379. doi: 10.1248/cpb.54.1370. [DOI] [PubMed] [Google Scholar]
- 4.Ghani N. Khazaenul Advia. 1st edition. New Delhi: Idarah Kitab ul Shifah; 1971. p. 1160. [Google Scholar]
- 5.Singh V, Jain AP. Ethnobotany and Medicinal Plants of India and Nepal. Vol. 1. Jodhpur: Scientific Publishers India; 2003. p. 145. [Google Scholar]
- 6.Kirtikar KR, Basu BD. Indian Medicinal Plants. 2nd edition. IX. Dehradun: Oriental Enterprises; 2003. pp. 2779–2781. [Google Scholar]
- 7.The Ayurvedic Pharmacopoeia of India. Part-1. II. New Delhi: Ministry of H & FW, Govt. of India, Department of Ayush; Anonymous; pp. 37–39. [Google Scholar]
- 8.Bahadur KD, Sen AB. Chemical Examination of Leucas cephalotes. Quarterly Journal of Crude Drug Research. 1969;9:1453–1454. [Google Scholar]
- 9.Medicinal Plants in Folklores of Bihar and Orissa. 1st edition. New Delhi: CCRUM, Ministry of H & F W Govt. of India; 2001. Anonymous; p. 314. [Google Scholar]
- 10.Robert A, Turner . Screening Methods in Pharmacology. London and New York: Academic press; 1965. pp. 229–300. [Google Scholar]
- 11.Singh N, Nath R, Singh DR, Gupta ML, Kohli RP. An Experimental Evaluation of Protective Effects of Some Indigenous Drugs on Carbon tetrachloride induced Hepatotoxicity in Mice and Rats. Quarterly Journal of Crude Drug Research. 1978;16:8–16. [Google Scholar]
- 12.Freirich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative Comparison of Toxicity of Anti-ulcer Agents in Mouse, Rat, Hamster, Dog, Monkey and Man. Cancer Chemotherapy Report. 1966;50(4):219–244. [PubMed] [Google Scholar]
- 13.Recknagel RO, Ghoshal AK. Quantitative Estimation of Peroxidative Degenera-tion of Rat Liver Microsomal and Mitochondrial Lipids after Carbon tetrachloride Poisoning. Experimental and Molecular Pathology. 1966;5:413. doi: 10.1016/0014-4800(66)90023-2. [DOI] [PubMed] [Google Scholar]
- 14.Dwiwedi Y, Rastogi R, Chander R, Sharma SK, Kapoor NK, Dhawan BN. Hepatoprotective Activity of Picroliv Against Carbon tetrachloride induced Liver Damage in Rats. Indian Journal of Medical Research. 1990;92:195–200. [PubMed] [Google Scholar]
- 15.Reitman S, Frankel AS. A Colorimetric Method for the Determination of Serum Glutamic Oxaloactic and Glutamic Pyruvic Transminase. American Journal of Clinical Pathology. 1957;28:53–56. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 16.Remington The Science and Practice of Pharmacy. 21st edn. Vol. 1. New Delhi: Lippincott Williams & Wilkins, B.I. Publications Pvt. Ltd; 2006. Anonymous; p. 1110. [Google Scholar]
- 17.Sherlock S, Dooley J. Diseases of the Liver and Biliary System. 10th edition. Oxford: Blackwell Science; 1997. pp. 1–20. [Google Scholar]
- 18.Ploa GI, Hewitt WR. Principle and Methods of Toxicology. 2nd edition. New York: Raven press; 1989. p. 199. [Google Scholar]
- 19.Sharma ML, Chandokhe N, Ghatak BJ, Jamwal KS, Gupta OP, Singh GB, et al. Pharmacological Screening of Indian Medicinal Plants. Indian Journal of Experimental Biology. 1978;16(2):228–240. [PubMed] [Google Scholar]
- 20.Evans WC, Evans D. Trease and Evans Pharmacognosy. 15th edtion. New Delhi: Elsevier, a division of Reed Elsevier India Private Ltd; 2008. pp. 414–415. [Google Scholar]


