Figure 1. Domain architecture and reaction scheme of Cph1.
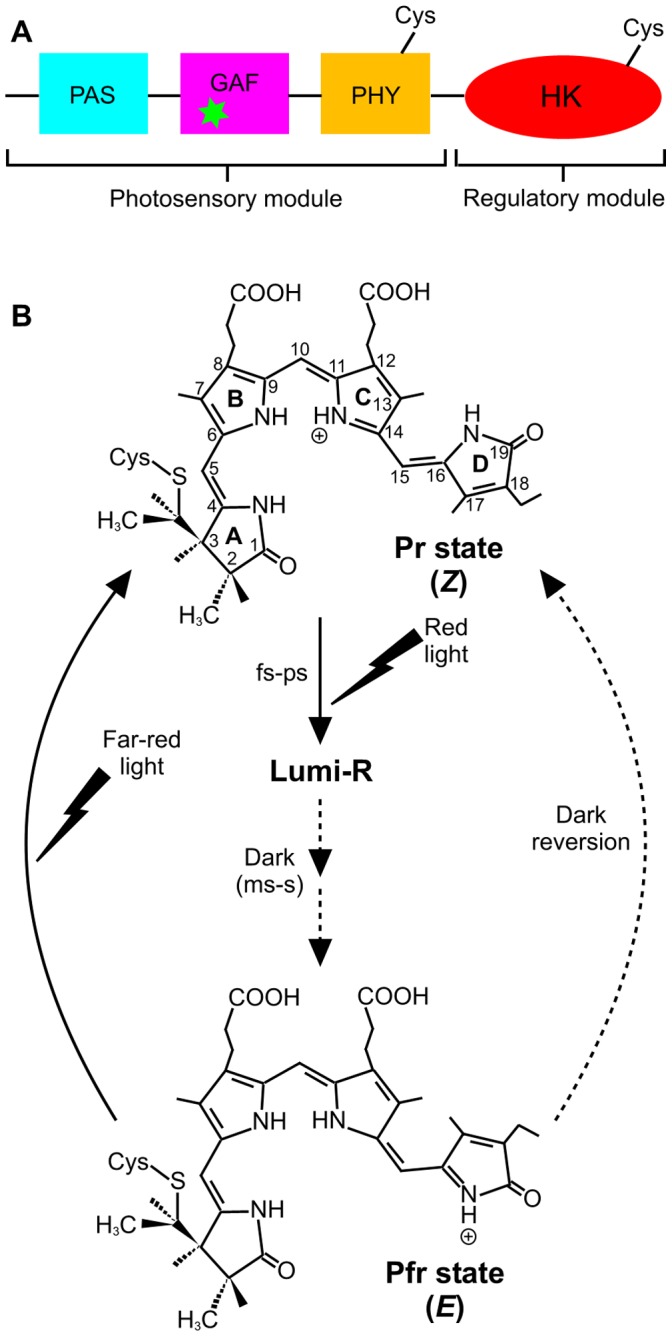
(A). Domain architecture of phytochrome, consisting of an N-terminal photosensory module that comprises the PAS, GAF and PHY domains, and a C-terminal regulatory module that contains a histidine-kinase like domain (HK) [1]. The Cys residues that have been used for spin-labeling studies in the present work are shown and the green asterisk indicates the phycocyanobilin cofactor. (B). The proposed photoconversion of the Pr to Pfr states of the phycocyanobilin cofactor in Cph1 phytochrome. The chromophore in the Pr state is shown as ZZZssa at the AB, BC, and CD rings, respectively [5]. Illumination with red light triggers photoisomerization about the C15–C16 methine bridge to give the primary photoproduct, Lumi-R, which is subsequently converted to Pfr in several light-independent steps on the millisecond-to-second timescale. The Pr state can be regenerated from Pfr by excitation with far-red light or by a slow dark reversion process [1].
