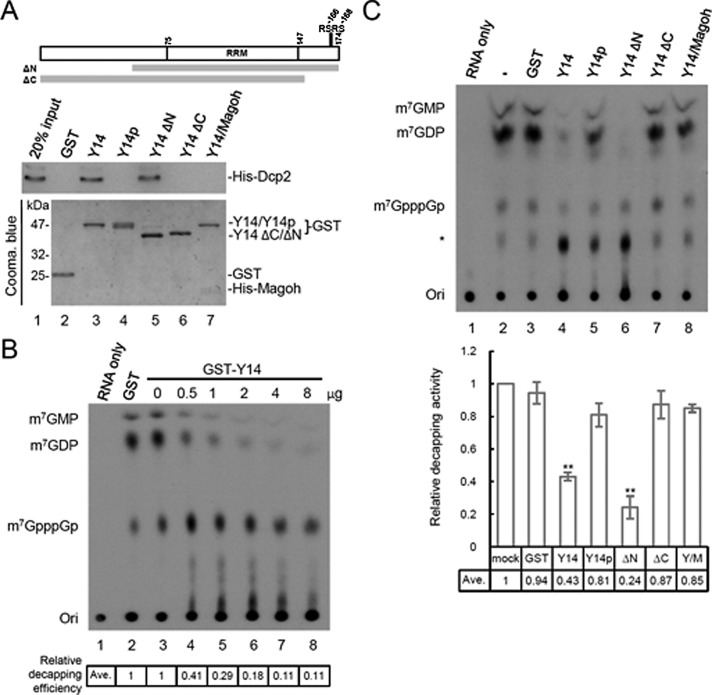
FIGURE 2:
Y14 interacts directly with Dcp2 and inhibits its decapping activity. (A) Diagram shows the domains of full-length Y14 and truncated forms of Y14. Y14 can be phosphorylated at the serine residues of RSRS near the C-terminus. Recombinant GST or one of the various GST-Y14 proteins (including the full-length nonphosphorylated and phosphorylated Y14, Y14∆N, or Y14∆C, and GST-Y14/His-Magoh) was incubated with His-Dcp2, followed by affinity chromatography using glutathione–Sepharose. Sepharose-bound His-Dcp2 was detected by immunoblotting using anti-Dcp2. The bait proteins were detected by Coomassie blue staining. (B) The decapping reaction was performed by incubating His-Dcp2 and GST (lane 2) or increasing amounts of GST-Y14 (lanes 3–8) with a 176-nucleotide RNA containing a 32P-labeled 5′ cap and then analyzed by TLC. Lane 1 shows RNA only; ori indicates the origin of the TLC plate. Relative decapping efficiency is indicated below the gel. (C) The Dcp2 decapping assay was performed as in B; the reactions did not contain any additional recombinant protein (lane 2) or contained GST (lane 3), GST fused to full-length or truncated Y14 (lanes 4–7; as in A), or GST-Y14/His-Magoh (lane 8). Asterisk indicates an unidentified nucleotide; its level varied among different batches of recombinant Y14 proteins. The bar graph shows relative inhibition of decapping for the different GST-Y14 forms; the mean and SD were obtained from three independent experiments, and asterisks indicate p < 0.01.