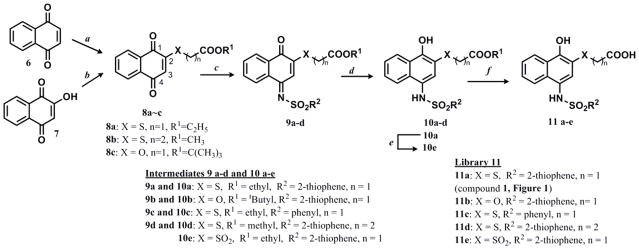
Scheme 2.
Synthetic route to compound 1 (hit) and derivatives of the hit
Reagents and conditions: a) HS(CH2)nCOOR1 (R1 = ethyl, n =1 for 8a, 89 % and R1 = methyl, n =2 for 8b, 94 %), ethanol (2.5 mL/mmol), r.t. 0.5 h; b) BrCH2COOtBu, Ag2O, CHCl3, cat. KI, reflux under Ar, 36 h, 33%; c) R2SO2NH2, TiCl4·2THF, Et3N, DCM, microwave, 60 °C, 5~20 min, 26~62%; d) Na2S2O4, THF, 1 h, r.t. or EtOAc, H2O, r.t. 10 min., 59–93%; e) Oxone, H2O, acetone, r.t., 12–14 h, 100%; f) conc. HCl/dioxane (1:1, 16 mL/mol), r.t 3–36 h or microwave, 100 °C, 10 min. 54–97%.