Abstract
A family of methacrylic terpolymer biomaterials was electrospun into three-dimensional scaffolds. The glass transition temperature of the polymer correlates with the morphology of the resulting scaffold. Glassy materials produce scaffolds with discrete fibers and large pore areas (1531±1365 μm2), while rubbery materials produce scaffolds with fused fibers and smaller pore areas (154±110 μm2). Three different endothelial-like cell populations were seeded onto these scaffolds under static conditions: human umbilical vein endothelial cells (HUVECs), adult human peripheral blood-derived outgrowth endothelial cells, and umbilical cord blood-derived human blood outgrowth endothelial cells. Cellular behavior depended on both cell type and scaffold topography. Specifically, cord blood-derived outgrowth endothelial cells showed more robust adhesion and growth on all scaffolds in comparison to other cell types as measured by the density of adherent cells, the number of proliferative cells, and the enzymatic activity of the adherent cells. Peripheral blood-derived outgrowth cells exhibited less ability to inhabit the terpolymer interfaces in comparison to their cord blood-derived counterparts. HUVECs also exhibited less of a capacity to colonize the terpolymer interfaces in comparison to the cord blood-derived cells. However, the mature endothelial cells did show scaffold-dependent behavior. Specifically, we observed an increase in their ability to populate the low-porosity scaffolds. All cells maintained an endothelial phenotype after 1 week of culture on the electrospun scaffolds.
Introduction
Many blood-contacting biomedical devices fail due to thrombus development (blood coagulation), which occurs at the blood–biomaterial interface.1 Examples of this include occlusion of small diameter vascular grafts and impaired movement of artificial heart valve leaflets, making blood–material interactions one of the most pressing problems in the field of biomaterials.2–5 Native vasculature is lined with a monolayer of endothelial cells (an endothelium) and this interface is responsible for the blood compatibility of the vasculature.1 Many researchers have attempted to create material systems that foster a functional endothelial cell layer to generate a blood compatible surface6–8; however, no material has found clinical success. As such, developing strategies to improve the adhesion and growth of endothelial cells on biomaterial interfaces is an active research area.9,10
Most of this research focuses on enhancing the adhesion and growth of mature and terminally differentiated endothelial cells to a substrate.6–10 However, in addition to mature endothelial cells other endothelial-like cell sources exist.11,12 In particular, we are interested in the capacity of outgrowth endothelial cells (OECs) to endothelialize a biomaterial. OECs are derived from stem/progenitor cells found in peripheral blood. When these cells are appropriately cultured in vitro, colonies of cells with the endothelial phenotype are produced, yet these cells also retain the increased proliferation capacities and rates of undifferentiated cells.13 The culture of adult human peripheral blood-derived outgrowth endothelial cells (pbOECs) from circulating progenitor cells was first described by Asahara and colleagues in 1997 and has since been extensively studied due to their therapeutic potential.14–16 Similarly, umbilical cord blood-derived outgrowth endothelial cells (cbOECs) can also be obtained.17,18 In this article, we assess the capacity of three different endothelial cell populations to endothelialize electrospun scaffolds: mature endothelial cells (human umbilical vein endothelial cells [HUVECs]), pbOECs, and cbOECs.
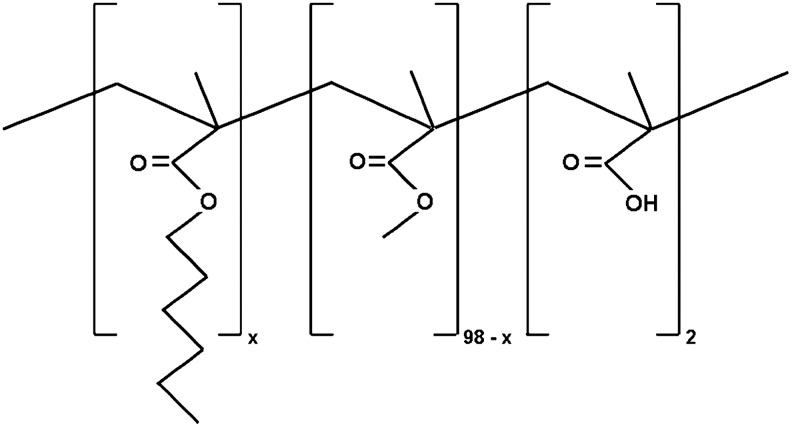
The biomaterial system used in this research is a methacrylic terpolymer produced through free radical copolymerization of hexyl methacrylate (HMA), methyl methacrylate (MMA), and methacrylic acid (MAA).19–27 The material contains 2 mole% MAA to allow postsynthesis derivatizations. The remaining 98 mole% is composed of HMA and MMA. By controlling the ratio of HMA:MMA in the material, we can tailor the physical properties of the material by controlling the glass transition temperature (Tg) of the polymer, enabling us to create a single biomaterial system with a wide range of physical properties.19,24 We developed this biomaterial family to generate biomaterials, which can be easily functionalized, and to allow facile changes to the mechanical behavior of the polymer. Polymer composition, molecular weight, polydispersity, water absorption, glass transition temperature, and tensile properties have been previously reported.24 Subsequently, the terpolymer system has been electrospun, and it was found that varying the glass transition temperature of the polymer allowed us to control the morphology of the resulting electrospun scaffold. In turn, this enabled us to probe the effect of scaffold topography on cellular behavior.23,25,26 Specifically, glassy materials produced scaffolds with a high porosity and discrete fibers, while rubbery materials produced scaffolds with much lower porosities and fused fibers.
In recent years, scaffold topography has emerged as a key factor in directing the behavior of adherent cells28–30; therefore, the above listed cells types were seeded onto electrospun scaffolds with different topographies to see if we observe scaffold-dependent cellular behavior. We hypothesized that the different cell types will show varying capacities to endothelialize the terpolymer biomaterial, and the electrospun scaffold topography will affect the ability of the cells to adhere and proliferate on the material. From this research, we aim to identify the best combination of cell type and scaffold properties to promote endothelialization of implanted biomedical devices.
Materials and Methods
Polymer synthesis
The synthesis of the methacrylic terpolymer was achieved through free radical polymerization as described in detail previously.19,24 Briefly, the monomers used in the reaction were n-HMA (Alfa Aesar, Ward Hill, MA), MMA (ACROS Organics, Pittsburgh, PA), and MAA (ACROS Organics). The polymerization reaction was performed in 100 mL dimethylformamide (DMF; Sigma-Aldrich, Milwaukee, WI) using 2, 2-azobisisobutyronitrile (AIBN; Sigma-Aldrich) as the initiator. The molar ratios of HMA and MMA were varied to create polymer materials with different glass transition temperatures. In all cases, the amount of MAA was held constant at 2 mole%. The mass of monomer and initiator were held constant at 20 and 0.0040 g, respectively. The reaction was carried out for 48 h at 55°C–60°C in an inert argon atmosphere. The reaction was stopped by cooling, and the polymer was recovered through precipitation in an excess nonsolvent (an equivolume mixture of distilled water and methyl alcohol). The polymer was dried in an oven at 55°C–60°C for 48 h, and then kept in a desiccator.
Preparation of electrospun terpolymer scaffolds
Terpolymer fiber samples were produced by electrospinning as previously described.24,25 Briefly, the terpolymer was dissolved in an equimolar mixture of ether and acetone to a final concentration of 13% (g/mL). The solution was passed through a needle at a rate 16 mL/h using a syringe pump. The collector was placed at a distance of 16 cm from the needle. A voltage of 23 kV was applied between the needle and the collector. The polymer solution was drawn into fibers by the applied voltage and deposited on the collector in the form of nonwoven terpolymer mats. Fiber scaffolds were placed under vacuum overnight to remove the residual solvent. Cell experiments were begun within 1 week after fabrication of the electrospun scaffolds.
Scaffold characterization
The architecture of the random fiber scaffolds was examined using scanning electron microscopy (Philips XL30 ESEM FEG). Samples were sputter coated with gold and imaged using a 10-kV accelerating voltage. The fiber diameter and pore area of the terpolymer meshes were measured from the scanning electron micrographs by random counting of 150 fiber diameter and pore areas distributed evenly over three scaffolds using ImageJ image analysis software (ImageJ, Bethesda, MD).31 Pore area data were generated by measuring the area of the polygon created by the upper most layer of fibers. Fiber measurements were taken of the top layer of discrete fibers only.
Cell collection and culture
Human umbilical vein endothelial cells
HUVECs were obtained from American Type Culture Collection (ATCC, Manassas, VA). The cells were cultured according to standard procedure. Briefly, the medium used was Dulbecco's Modified Eagle's Medium (DMEM; Invitrogen) supplemented with 20% fetal bovine serum (FBS). The culture medium was changed every 3 days and cultures were passaged at 80% confluence to prevent contact inhibition. Cells at passage 6 were used in these experiments.
Cord blood HBOECs
Cord blood was purchased from the National Disease Research Interchange (NDRI, Philadelphia, PA). Upon arrival, the blood was incubated with RosetteSep Human CD45 Depletion Cocktail (Stemcell Technologies, Vancouver, British Columbus, Canada) at a ratio of 0.5 μL of cocktail per 1 mL of whole cord blood for 20 min while being gently agitated. Cord blood was diluted 1:1 with 2% FBS (Invitrogen, Carlsbad, CA) in phosphate-buffered saline (PBS; Invitrogen) and 20 mL of diluted blood was carefully layered onto 15 mL of Hystopaque-1077 (Sigma Aldrich) in a conical tube. Conical tubes were centrifuged for 20 min at 700 g at room temperature. The peripheral blood mononuclear cell-containing layer (the layer at the Hystopaque:plasma interface) was isolated and washed twice in 2% FBS+2 mM ethylenediaminetetraacetic acid (EDTA; Invitrogen) in the Hank's Balanced Salt Solution (HBSS; Invitrogen) to a volume of 50 mL and spun at 1200 rpm for 10 min at room temperature to reisolate cells. After the first washing step, 5–10 mL of ammonium chloride was added, and cells were incubated for 10 min to lyse residual nucleated blood cells. After the second washing step, cells were resuspended in the endothelial growth medium-2+10% FBS (Lonza, Walkersville, MD). Five million cells were plated per well of a 12-well plate precoated with type-1 collagen (BD, Franklin Lakes, NJ). The medium was changed every day for the first 7 days and every other day after that. Colonies of outgrowth endothelial cells (OECs) were observed between 5–10 days after initial plating. Approximately 3–5 days after initial formation of OEC colony, cells were lifted with 0.05% Trypsin/EDTA (Invitrogen) and reseeded into 2–3 collagen-coated wells of a 12-well BD Falcon multiwell plate. After reseeding, cells were trypinsinized and split upon reaching 70%–90% confluence. Cells continued to proliferate rapidly up to passage 10–12; however, cells at passage 4 were used for these experiments.
Peripheral blood HBOECs
About 60 mL of peripheral blood was obtained from a healthy 30-year-old male volunteer. The procedure for PBMC isolation from adult peripheral blood was the same as for cord blood with the following changes: the RosetteSep cocktail was not used, cells were washed 3× in 2% FBS+2 mM EDTA in HBSS without the use of ammonium chloride, and 20 million PBMCs were plated per well of a six-well plate coated with type-1 collagen. Outgrowth endothelial colonies (pbOECs) were observed between days 10–18 after initial plating. Approximately 5–7 days after the initial formation of the OEC colony, cells were lifted with 0.05% Trypsin/EDTA and reseeded into 1–3 collagen-coated wells of a six-well plate. After reseeding, cells were trypsinized and split upon reaching 70%–90% confluence. Cells continued to proliferate rapidly up to passage 5–6; however, cells at passage 4 were used for these experiments.
All cultures were maintained in a humidified incubator at 37°C and 5% CO2.
Preparation of cell culture surfaces
Cell experiments were performed in tissue-culture-treated 24-well plates (BD Falcon). The cell growth surfaces were first coated with a film of the desired polymer through solution casting of a 5% (g/mL) solution of the desired polymer in THF. The electrospun meshes of the appropriate composition were cut to size using a biopsy punch. Since some of the scaffolds are highly porous, precoating the film of polymer ensures that if cells are capable of penetrating the scaffold they will interact with a film of the polymer instead of the growth surface of the well plate. Wells containing only solution-cast films of polymer were used as negative controls, while unmodified wells of tissue culture polystyrene (TCPS) were used as the positive control. Well plates were sterilized by exposure to UV radiation for a minimum of 2 h, submerged in PBS, and allowed to equilibrate overnight.
Cells were lifted using 0.25% trypsin/EDTA, counted with a hemocytometer, centrifuged, old media was aspirated, and cells were diluted to the desired density through the addition of fresh media. About 15,000 cells were added to each well (7500 cells/cm2), the medium was changed every 2 days, and cells were allowed to interact with the biomaterials for the desired length of time.
Adhesion and proliferation of endothelial cells
Cells were incubated with the surface of interest for 5 days. On days 1 to 4, cultures were inoculated with 5-ethynyl-2′-deoxyuridine (EdU) (Click-iT EdU Alexa Fluor 594 imaging kit; Invitrogen) according to manufacturer's instructions. EdU is a reagent, which is incorporated into a cell's DNA during the s-phase of cellular division.32 At the end of the culture period, cells were rinsed three times with prewarmed Dulbecco's phosphate-buffered saline (DPBS), fixed for 1 h in 3.7% formaldehyde (FA) at room temperature, permeabilized with 0.1% Triton X-100 for 30 min, fluorophores were covalently attached to the DNA-bound EdU by using the Click-iT reagents, cells were stained with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen), and mounted using the Prolong antifade reagent (Invitrogen). The adherent cells were observed with fluorescence microscopy. When imaging flat surfaces (for instance, the TCPS-positive control), brightfield fluorescence microscopy was used. When imaging more three-dimensional surfaces (e.g., the electrospun scaffolds), confocal microscopy was used to generate a z-stack, which enables simultaneous viewing of cells on multiple focal planes.
Quantification of cell adhesion
Ten randomly selected and nonoverlapping images were taken on each biomaterial. The cell density was measured by counting the number of DAPI-stained nuclei per image and dividing that number by the area of the image. The experiment was performed in triplicate for a total of 30 images spread evenly over three test surfaces per type of biomaterial.
Quantification of cell proliferation
Five randomly selected and nonoverlapping images were taken on each biomaterial. The number of DAPI-stained nuclei and the number of EdU-stained nuclei per frame were counted and recorded. The percent of cells that were stained red by EdU was calculated as an assessment of cell proliferation. The experiment was performed in triplicate for a total of 15 images spread evenly over three test surfaces per biomaterial.
For both adhesion and proliferation studies, images taken at 10× magnification were used for the quantification.
Enzymatic activity of endothelial cells
As an assessment of cell viability, the enzymatic activity of the adherent cells was measured using the Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Gaithersburg, MD). This is a colorimetric assay based on the cleavage of a tetrasolium salt (WST-8) by mitochondrial dehydrogenases in viable cells. Increased enzyme activity leads to an increased dye formation that is measured using a spectrophotometer.
Culture plates were prepared as described above. After a 7-day incubation period, the cell culture medium was aspirated and a fresh medium was added along with the CCK-8 reagent (10:1 ratio). Plates were then incubated for 3 h in an incubator. After incubation, 100 μL of solution from each well was transferred into three wells of a 96-well plate, where the absorbance of the solution was measured at 450 nm. Wells containing a medium and the CCK-8 reagent without cells were used to adjust for background signal. The experiment was repeated in quadruplicate.
Phenotype of endothelial cells
Endothelial cells were allowed to incubate with the surface of interest for 5 days. On the final day of culture, cells were stained for the surface affinity of FITC-labeled lectin (Ulex Europaeus) according to manufacturer's instructions (Sigma). After 1 h of incubation with the staining solution, cells were rinsed three times with prewarmed DPBS, fixed for 1 h in 3.7% FA at room temperature, and mounted using the Prolong antifade mounting agent. Samples were imaged using either bright field fluorescence or confocal microscopy.
Statistical analysis
JMP data analysis software was used to perform the statistical analyses. When more than two means were compared, analysis of variance (ANOVA) was used to determine if any pairs of means were statistically different. If the ANOVA indicated statistical difference, then the Tukey–Kramer Honestly Difference Test was used to determine which of the pairs of means were statistically different in all tests α<0.05.
Results
Polymer characterization
As described in the Introduction, a family of methacrylic terpolymer was the biomaterial system used in this research. A schematic of the polymer system and its composition is shown in Figure 1. From this figure, we can see that all materials contain ∼2 mol% MAA and the remaining 98 mol% is either HMA or MMA which enables control of the physical properties of the material through variation of the polymer's glass transition temperature (Tg). Throughout the text, the polymer system will be referred to by the amount of HMA used in its synthesis. For instance, H30 refers to a material copolymerized from a feed containing 30/68/2 mol% HMA/MMA/MAA, while H80 refers to a material copolymerized from a feed containing 80/18/2 mol% HMA/MMA/MAA. For this work, we chose to study electrospun scaffolds of H30, H70, and H80. The glass transition temperature and the room temperature film modulus of the terpolymer biomaterials are compiled in Table 1. From the table, we can see that the glass transition temperature as well as the modulus of the polymer decreases with increasing HMA content (for details on polymer characterization, please see Ref.24).
FIG. 1.
Schematic of terpolymer molecular composition.
Table 1.
Characterization of Polymer and Electrospun Scaffolds: Glass Transition Temperature, Film Modulus, Scaffold Fiber Diameter, and Scaffold Pore Area
| Biomaterial (–) | Tg (oC) | Film modulus (MPa) | Fiber diameter (μm) | Pore area (μm2) |
|---|---|---|---|---|
| H30 | 59±4 | 295±46 | 7.68±2.99 | 1531±1365 |
| H70 | 12±2 | 18±14 | 4.46±1.28 | 388±276 |
| H80 | 3±4 | 15±5 | 4.78±1.57 | 154±110 |
Values are reported as average±standard deviation. 150 data points were analyzed per fiber diameter and pore area average.
Tg, glass transition temperature.
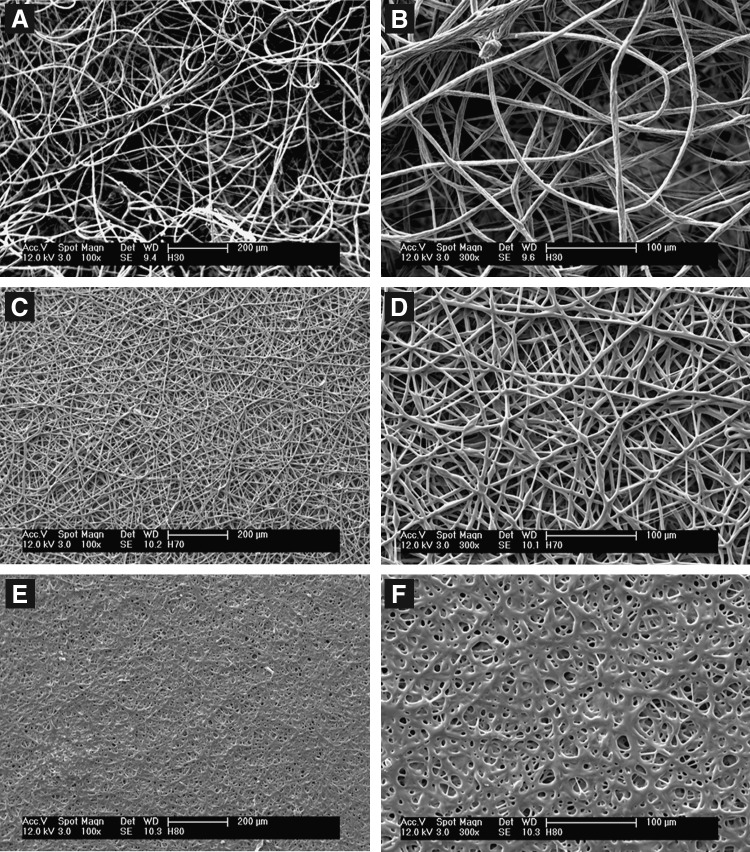
Figure 2 contains scanning electron micrographs of the scaffolds used in this work. Low-magnification images (panels A, C, and E) allow the topography of the scaffolds to be observed, while higher magnification images (panels B, D, and F) allow observation of the individual fibers. From these images, we observe large changes in electrospun scaffold topography and fiber surface roughness with changes in the polymer composition. Specifically, the fibers in the H30 scaffolds are discrete and possess rough surfaces and the scaffold contains large pores (panels A and B), while the fibers in the H80 scaffolds are fused and smooth and the scaffolds contain much smaller pores (panels E and F).
FIG. 2.
Scanning electron micrographs of terpolymer scaffolds of various chemical compositions: (A) H30 low magnification, (B) H30 high magnification, (C) H70 low magnification, (D) H70 high magnification, (E) H80 low magnification, and (F) H80 high magnification. When originally sized low-magnification images are at 100× magnification and high-magnification images are 300× magnification.
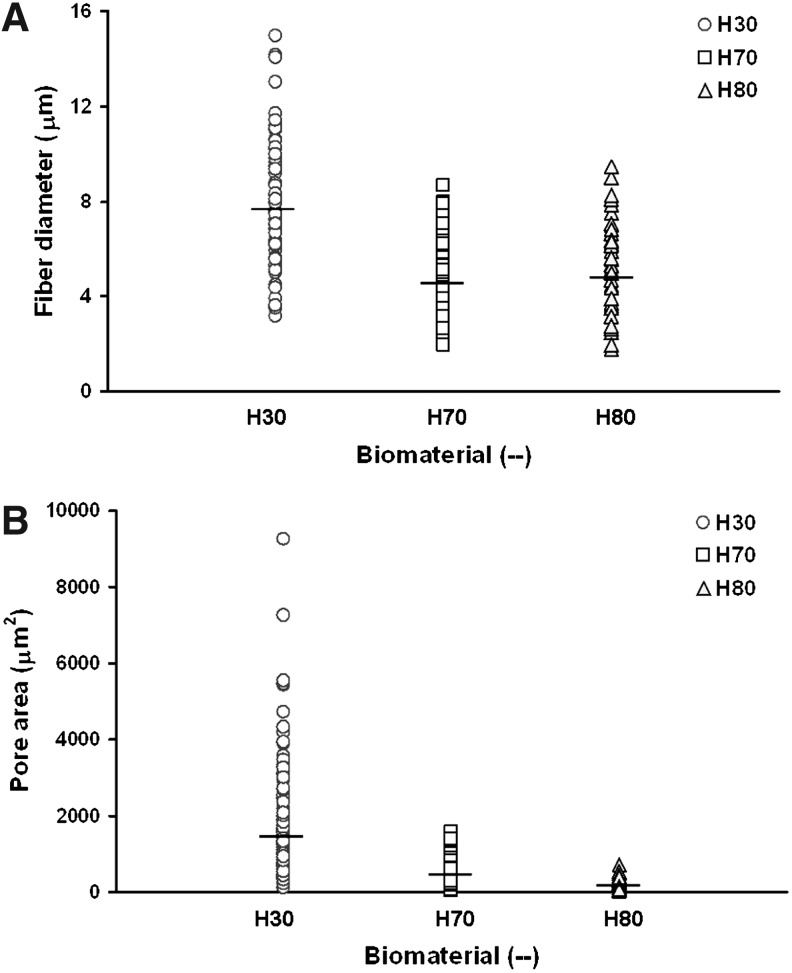
Scanning electron micrographs of the scaffolds were quantified and scatter plots of the fiber diameter and pore size were constructed (Fig. 3). Horizontal hash marks in Figure 3 represent the average of these data. The fiber diameter and pore size averages are compiled in Table 1. From the scatter plots, we can observe that the distribution of both the fiber diameter and pore size data are uni-modal; however, the data for the H30 scaffolds span a greater range and contain more high-end outliers indicating that the H30 scaffolds contains greater variation in its structure than the H70 and H80 scaffolds. The average diameter of H70 and H80 fibers are very similar; however, the H30 polymer produced fibers with larger fiber diameters. Although fiber diameters in the H70 and H80 scaffolds are very similar, the average pore area in the H70 scaffolds is more than twice than that found in the H80 scaffold, while the H30 scaffold has an average pore area 10 times the average pore area found in the H80 scaffolds.
FIG. 3.
Scatter plots of (A) fiber diameter and (B) pore area data collected from quantification of scanning electron micrographs. About 150 fiber diameter and pore area data points were collected per biomaterial. Horizontal hash marks indicate the averages of these data.
Biological interactions
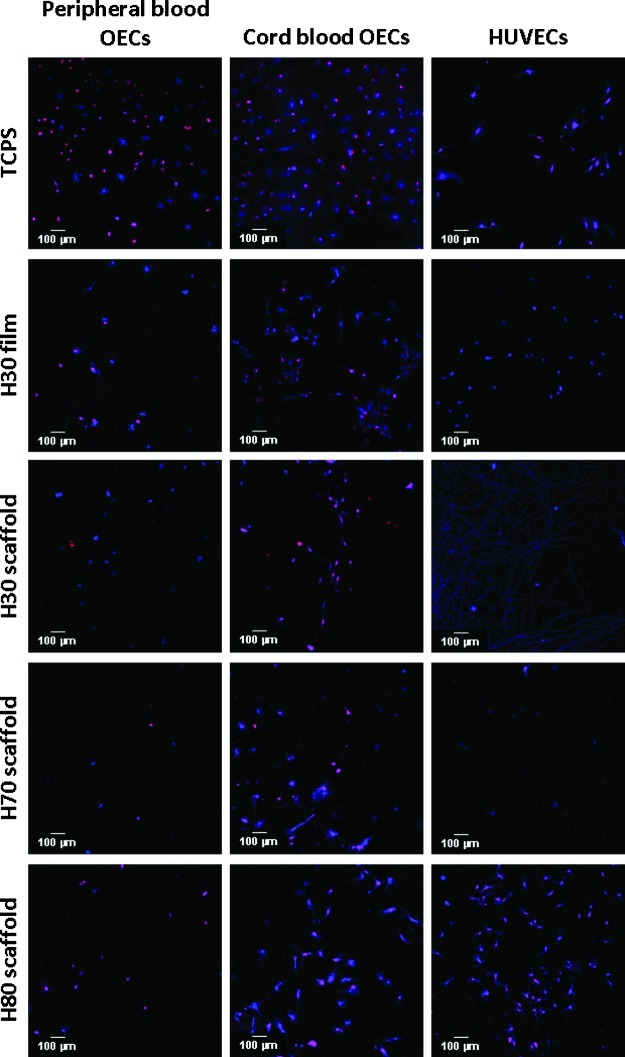
The H30, H70, and H80 terpolymer scaffolds were seeded with one of three populations of endothelial-like cells: pbOECs, cbOECs, and HUVECs. H30 films were used as the negative control, while TCPS was used as the positive control in cellular experiments. Cultures were stained with both DAPI and EdU and imaged using fluorescence microscopy. Figure 4 provides representative images from each culture condition. From these images, we can see that pbOECs exhibited low cellular adhesion and proliferation on all the terpolymer surfaces. Similarly, HUVECs showed low adhesion and proliferation on the H30 and H70 terpolymer scaffolds. Interestingly, the H80 scaffold appears to foster a larger population of more highly proliferative HUVECs. All biomaterials were inhabited by a higher density of cbOECs in comparison to other cell types and a large population of these cells stained positive with EdU indicating high proliferation rates.
FIG. 4.
Fluorescence microscopy images of endothelial cell nuclei after 5 days of incubation on terpolymer scaffolds. DAPI (blue) stains all cells within the electrospun scaffold, while EdU (red) indicates cells with active DNA synthesis during the incubation period. DAPI, 4′,6-diamidino-2-phenylindole; EdU, 5-ethynyl-2′-deoxyuridine; HUVECs, human umbilical vein endothelial cells; OECs, outgrowth endothelial cells; TCPS, tissue culture polystyrene. Color images available online at www.liebertpub.com/tea
Fluorescence images were quantified to determine the total number of adherent cells on each biomaterial and the total number of proliferative cells on each biomaterial. Figure 5 is a bar chart that compiles the density of adherent cells on each biomaterial.
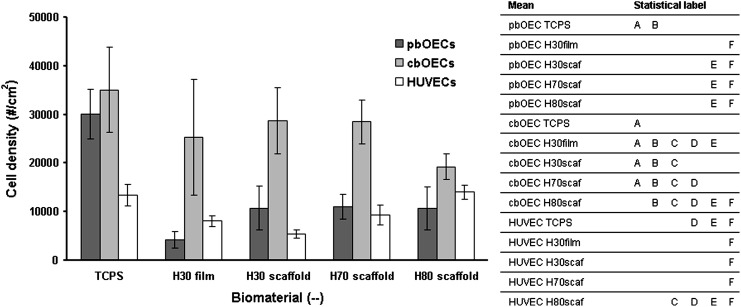
FIG. 5.
Quantification of adherent endothelial cell density on terpolymer scaffolds after 5 days of incubation. To the right of the bar chart, you will find a table that denotes statistical differences. Means in the chart which are labeled with the same letter are not significantly different. For instance, the density of adult OECs on TCPS and cord OECs on TCPS are not statistically different because they are both labeled with A. pbOECs, peripheral blood-derived outgrowth endothelial cells; cbOECs, cord blood-derived outgrowth endothelial cells.
The images presented in Figure 4 are corroborated by the quantification presented in Figure 5. From Figure 5, we see that the population of cbOECs on TCPS is high. Furthermore, the cbOEC cell density on most of the terpolymer surfaces is statistically the same as the positive control. The population of pbOECs on TCPS is high; however, much lower densities of pbOECs were observed on the terpolymer biomaterials. The mature endothelial cell population (HUVECs) has a consistently low surface density. However, HUVECs on the H80 surface are the only population, which is statistically the same as the cbOECs possibly illustrating their increased ability to inhabit the H80 scaffold.
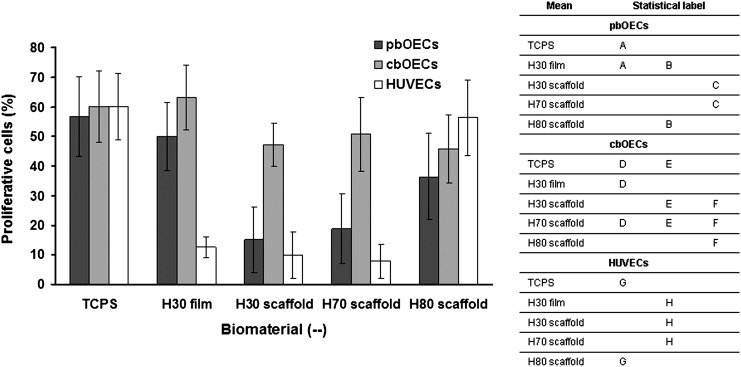
Proliferation data are compiled in Figure 6. When performing the statistical analysis on these data, only the averages for each cell type were compared, since the division rates of these cell populations differ.13,22 From these data, we see that ∼60% of all cells, regardless of the cell type, participated in cellular division on the TCPS during the incubation period. A high percentage of the cbOECs participated in cellular division on all biomaterials, while the pbOECs on the terpolymer scaffolds displayed suppressed levels of cellular division. HUVECs also showed consistently lower levels of cellular division except on the H80 scaffold, where a much greater percentage of nuclei are stained red.
FIG. 6.
Quantification of endothelial cell proliferation on terpolymer scaffolds after 5 days.
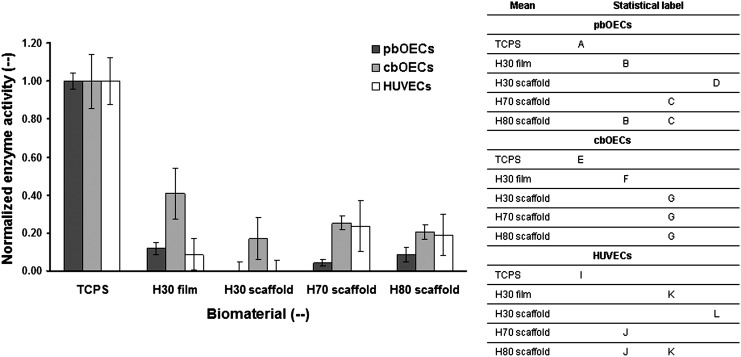
Cell viability was probed through a colorimetric enzyme activity assay. In these tests, absorbance was proportional to the enzymatic activity of the culture. As displayed in Figure 7, for each cell type, the data were normalized by the positive control. In the statistical analysis, only averages for each cell type were compared as each distinct cell population may have different enzyme activity. The data from this experiment corroborate the results presented above. As expected, a higher enzyme activity from cells cultured on the positive control was observed. However, on the terpolymer biomaterials, the highest enzyme activity was observed from the cbOECs. HUVECs and pbOECs displayed a relatively low enzyme activity except for on the low-porosity terpolymer scaffolds, where the HUVECs showed enhanced enzyme activity.
FIG. 7.
Enzymatic activity of adherent endothelial cells after 7 days of culture.
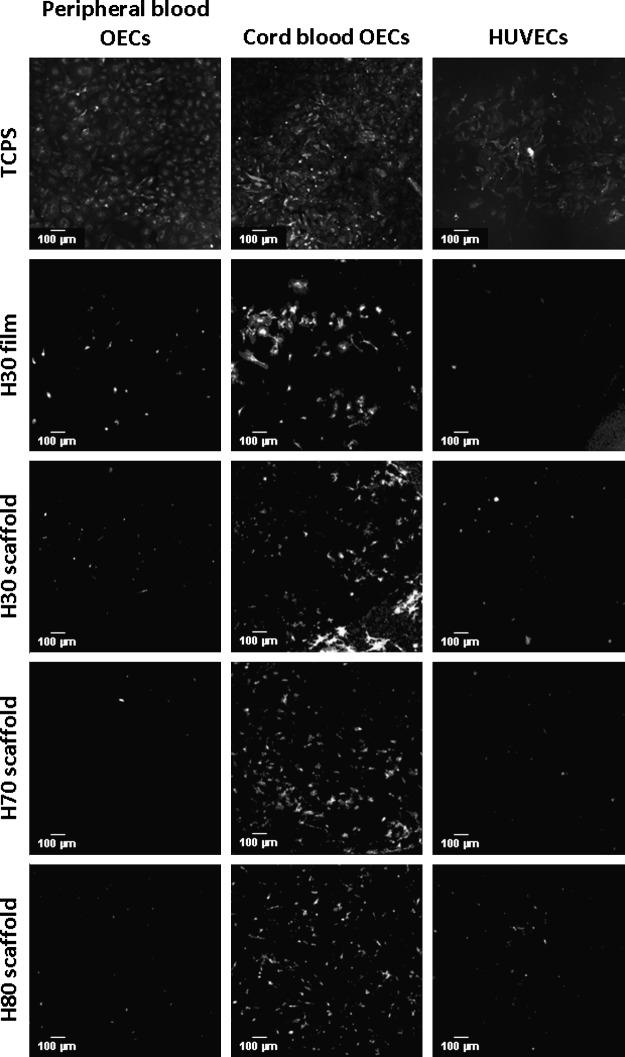
The phenotype of the cultured cells was also probed through the use of fluorescence microscopy by staining for the membrane affinity for lectin. From these images, we see cbOECs have formed a confluent monolayer on the TCPS, while the HUVECs have not proliferated sufficiently to cover the entire growth surface of the well (Fig. 8). As expected, few pbOECs and HUVECs are found adherent to the biomaterials; however, a higher population of cbOECs is found in all terpolymer surfaces. The endothelial-like cells stain positive for lectin indicating the retention of the endothelial phenotype.
FIG. 8.
Fluorescence microscopy images of lectin-stained cells after 5 days of culture on terpolymer scaffolds.
Discussion
As illustrated by the data in Table 1, terpolymer richer in HMA has a suppressed glass transition temperature and a lower modulus due to the long and linear pendant group displayed by the HMA repeat unit.33,34 The ability to control the mechanical behavior of the polymer by varying the composition of the polymer allows this family of polymers to be used in a wide variety of biomaterial applications.35–37
Interestingly, we also observed that controlling the Tg of the polymer enables scaffold morphology to be tailored. Specifically, glassy materials (H30) produce scaffolds with a high porosity and large pores with discrete and rough fibers, while rubbery materials (H80) produce scaffolds with a much lower porosity and pore size with fused and smooth fibers. The glass transition temperature of a polymer is molecularly interpreted as the temperature at which polymer chains begin long range backbone rotations providing materials above their Tg increased ability to relax residual stresses incorporated through fabrication processes.33,34 This molecular motion available to rubbery materials results in the H70 and H80 scaffolds dissipates internal stresses created during the electrospinning process, resulting in fiber contraction and a more porous and a more film-like appearance compared to the glassy H30 material. The data presented in Figure 3, which shows the thickness of fibers in the electrospun mats were taken from the top layer of discrete fibers only. It must be pointed out that fiber fusion occurred in the lower levels of the H80 scaffold resulting in larger features.
This rationalization is supported by previous studies. Many synthetic polymers, such as poly(ɛ-caprolactone); polystyrene; polymethyl methacrylate; polylactide, polyglycolide, and their copolymers; poly(ethylene oxide); and poly(dimethyl siloxane) (PDMS) have been fabricated into fibrous scaffolds by electrospinning.38–43 All of these materials are either glassy or semicrystalline at room temperature except PDMS.44 Furthermore, electrospun scaffolds of these polymers all result in scaffolds with discrete fibers and highly porous structures except PDMS–a rubbery material–which resulted in scaffolds with relatively low porosities and fused fibers similar to what is observed in the rubbery terpolymers we have electrospun in this work. These previous studies corroborate our interpretation that materials above their glass transition temperature and which lack crystallinity tend to produce these low-porosity scaffolds with fused fibers. However, according to the author's knowledge, we are the first to directly study the affect of Tg on electrospun scaffold topography.
Due to the ease in which we can control the morphology of the terpolymer scaffolds, this family of materials provides a facile method of exploring how scaffold topography affects the behavior of adherent cells. Specifically, these three terpolymer compositions were chosen for use in this study because they enable us to create scaffolds with a wide range of pore sizes. However, it must be pointed out that differences in cellular behavior on these scaffolds cannot be fully attributed to scaffold topography. Surface chemistry and modulus are also changing with the scaffold type, and both of these factors have been illustrated to affect the cellular behavior of adherent cells.26,36 Also, we expect the rubbery H70 and H80 scaffolds to coalesce into a film-like structure over large time scales due to the viscoelastic nature of the material. This limits the utility of terpolymer scaffolds to short-term applications as the desired topographical cues will slowly degrade over time.
In this research, we chose to explore the capacity of three different endothelial-like cell types to inhabit a biomaterial interface: HUVECs, pbOECs, and cbOECs. Mature endothelial cells, such as HUVECs are the standard cell source used in endothelialization experiments,6–10 and pbOECs are currently being studied for their therapeutic potential in patients with cardiovascular disease (CVD).14–16 However, most individuals with CVD are part of an aging population and it has been illustrated that the number of circulating endothelial progenitor cells drops with increasing age, limiting their use in the treatment of aging individuals. However, cord blood—if preserved—provides a large quantity of the patient's own highly proliferative stem cells which could be used in such personalized medicine endeavors. Furthermore, other recent work has illustrated phenotypic differences between cbOEC and pbOEC populations illustrating a need to further explore the differences between these two stem cell-derived cell types.28
To successfully endothelialize a medical implant, endothelial cells must be able to both adhere and proliferate on the surface. To probe the density of cells on the biomaterial surfaces, we quantified the number of adherent cells on each biomaterial after 5 days of incubation (Fig. 5). From these data, we found larger numbers of cbOECs on each biomaterial indicating that the cord blood-derived cells have the greatest capacity at inhabiting the biomaterial interfaces in comparison to the other cell types.
The data presented in Figure 5 only allow us to compare the numbers of adherent cells; however, the adherent cells must possess the ability to proliferate on the biomaterial interface. Therefore, EdU staining was used to assess cellular proliferation on the biomaterial surfaces (Fig. 6). In addition to the high cell density on the terpolymer scaffolds, we found that a large population of the cbOECs on the biomaterials also stained positive for proliferation indicating that this cell type was capable of both adhesion and growth on a variety of scaffold types. On most scaffolds, the pbOECs and HUVECs showed low levels of proliferation indicating that the cells present after 5 days of culture were from initial cell adhesion and not proliferation. Both HUVECs and pbOECs showed scaffold-dependent proliferation. Specifically, pbOECs on the H30 film and the H80 scaffold showed higher proliferation rates than on the H30 scaffold and the H70 scaffold indicating that these cells may prefer a more film-like environment for growth. We only observed increased HUVEC proliferation on the H80 scaffold.
Both the cell density and proliferation data presented above were found through the analysis of fluorescence images. To independently confirm the above data, we employed an enzymatic assay to measure cellular viability (Fig. 7). The enzymatic activity of cbOECs on all scaffold types was the closest to the positive control. Low levels of enzyme activity were observed for both the pbOECs and HUVECs, except for the HUVECs on the rubbery H70 and H80 scaffolds further indicating that the low-porosity scaffolds provide a more favorable culture environment for the mature endothelial cells.
Cell attachment to blood-contacting biomedical devices is often regulated by integrins present on the cell surface, which bind to ligands present at the biomaterial interface in a layer of proteins, which adsorb from serum (in vitro) or plasma (in vivo). Cell adherence to these binding sites is further complicated by the conformation of the adsorbed protein, which may change depending on both the surface chemistry and nanostructure of copolymer surfaces.45,46 It seems plausible that differences in integrin expression between cbOECs, pbOECs, and HUVECs result in the enhanced ability of the cord blood-derived cells to adhere to the biomaterials used in this study. However, the expression of molecules on the cbOEC surface in comparison to the other cell types has not been fully explored making it difficult to postulate a mechanism by which the improved cell adhesion occurs. However, the attachment of cbOECs from flow has been shown to be heavily dependent on their high expression of the α5β1 integrin, which is likely downregulated in the more differentiated pbOECs and HUVECs.47,48 Furthermore, pbOEC express larger amounts of integrin co-factor CYR61, and this molecule promotes cell adhesion. Furthermore, this molecule has been implicated in cell proliferation that may also help explain the increased proliferation of the cbOECs observed in this research.49
It was also observed that HUVECs showed enhanced proliferation on the low-porosity scaffolds. In native vasculature, endothelial cells are present on the luminal surface in a confluent monolayer and attached to an underlying basal lamina.50 These cells have spread morphology and are elongated in the direction of blood flow due to the shear stress of the passing fluid.51–53 Although the basal lamina is a fibrous structure composed primarily of elastin and collagen, these protein fibers often have nanometer fiber diameters.54 However, in this study, HUVECs are interacting with fibers and pores on the same size scale as the cells. One could envision the large gaps present in the high-porosity fibers may isolate individual cells from one another, preventing cell–cell interactions, and hindering cellular processes. On the other hand, the low-porosity scaffolds presented cells with a relatively flat surface allowing for firm attachment, growth, and spreading in a two-dimensional monolayer, yet also provided an increased surface area and topography, which may allow the cells to more easily attach and spread in comparison to a flat film. Such a structure that increases the attachment area available for the HUVECs, yet still enables the cells to grow in a two-dimensional monolayer, may result in the improved cellular behavior observed on the low-porosity scaffolds.
In this study, we were interested in determining the most effective cell source and scaffold type for the autologous endothelialization of biomedical materials. However, in addition to being able to inhabit the interface, it is important to determine that the cells retain the correct phenotype. Therefore, cultures were stained for the membrane affinity of lectin as described elsewhere.55–57 From the images presented in Figure 8, it can be seen that all adherent cells strongly stain positive for lectin indicating that the stem cell-derived populations of endothelial-like cells retained their phenotype after 5 days of culture on the terpolymer scaffolds.
Conclusion
The focus of this study was to determine if a particular source of endothelial-like cells possessed a greater capacity at inhabiting a biomaterial interface. Furthermore, we wished to assess if the scaffold type was capable of modulating the behavior of the adherent cells. The primary conclusion of this article is that cbOECs appear more capable of inhabiting and proliferating on a biomaterial surface than peripheral blood-derived outgrowth cells or HUVEC as measured through adherent cell density, proliferation, and enzymatic activity. Also, we observed consistent cbOEC behavior on all biomaterial interfaces indicating that the cbOECs have the capacity of endothelializing a variety of different surface structures. Additionally, it was observed that HUVECs exhibited scaffold-dependent behavior. Increased proliferation and enzyme activity of HUVECs adherent to the rubbery/low-porosity scaffolds was observed compared to the more fibrous scaffolds or films of polymer.
Acknowledgments
Funding for this research was supplied by NIH Grant RC2 AG036559. We would like to thank several individuals for their contribution to this work: Dr. Garland Fussell who did the initial work with this polymer system, Dr. Douglas Kniss who allowed generous access to his cell culture facility, and Dr. John Lannutti for access to his electrospinning facility.
Disclosure Statement
No competing financial interests exist.
References
- 1.Ratner B. Hoffman A. Schoen F. Lemons J. An introduction to materials in medicine. In: Ratner B., editor; Hoffman A., editor; Schoen F., editor; Lemons J., editor. Biomaterials Science. second. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- 2.Lamba N. Woodhouse K. Cooper S. Polyurethanes in Biomedical Applications. Boca Raton: CRC Press LLC; 1998. [Google Scholar]
- 3.Hench L. Biomaterials: a forecast for the future. Biomaterials. 1998;19:1419. doi: 10.1016/s0142-9612(98)00133-1. [DOI] [PubMed] [Google Scholar]
- 4.Ratner B. The catastrophe revisited: blood compatibility in the 21st century. Biomaterials. 2007;28:5144. doi: 10.1016/j.biomaterials.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bos G. Poot A. Beugeling T. van Aken W. Feijen Small diameter vascular graft prostheses: current status. Arch Phisiol Biochem. 1998;106:100. doi: 10.1076/apab.106.2.100.4384. [DOI] [PubMed] [Google Scholar]
- 6.Ling H. Garcia-Echeverria C. Asakura S. Sun W. Mosher D. Cooper S. Endothelial cell adhesion on polyurethanes containing covalently attached RGD-peptides. Biomaterials. 1992;13:905. doi: 10.1016/0142-9612(92)90113-3. [DOI] [PubMed] [Google Scholar]
- 7.Chung T. Lu Y. Wang S. Lin Y. Chu S. Growth of human endothelial cells on photochemically grafted Gly-Arg-Gly-Asp (GRGD) chitosan. Biomaterials. 2002;23:4803. doi: 10.1016/s0142-9612(02)00231-4. [DOI] [PubMed] [Google Scholar]
- 8.Dekker A. Beugeling R. Bantjes A. Feijen J. van Aken W. Adhesion of endothelial cells and adsorption of serum proteins on gas plasma-treated polytetrafluoroethylene. Biomaterials. 1991;12:130. doi: 10.1016/0142-9612(91)90191-c. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y. Tanaka M. Gong J. Yasuda K. Yamamoto S. Shimomura M. Osada Y. Platelet adhesion to human umbilical vein endothelial cells cultured on anionic hydrogel scaffolds. Biomaterials. 2007;28:1752. doi: 10.1016/j.biomaterials.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Yin M. Yuan Y. Wang J. Development of mussel adhesive polypeptide mimics coating for in-situ inducing re-endothelialization of intravascular stent devices. Biomaterials. 2009;30:2764. doi: 10.1016/j.biomaterials.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 11.Hirschi K. Ingram D. Yoder M. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duda D. Cohen K. Scadden D. Jain R. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc. 2007;2:805. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y. Weisdorf D. Solovey A. Hebbel R. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner J. Isolation of putative progenitor endothelial cells for angiogenisis. Science. 1997;275:964. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 15.Shantsila E. Watson T. Lip G. Endothelial progenitor cells in cardiovascular disease. J Am Coll Cardio. 2007;49:741. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 16.Zampetaki A. Kirton J. Xu Q. Vascular repair by endothelial progenitor cells. Cardio Res. 2008;78:413. doi: 10.1093/cvr/cvn081. [DOI] [PubMed] [Google Scholar]
- 17.Nagano M. Yamashita T. Hamada H. Ohneda K. Kimura K. Nakagawa T. Shibuya M. Yoshikawa H. Ohneda O. Identification of functional endothelial progenitor cells suitable for the treatment of ischemic tissue using human umbilical cord blood. Blood. 2007;110:151. doi: 10.1182/blood-2006-10-047092. [DOI] [PubMed] [Google Scholar]
- 18.Murohara T. Cord blood-derived early outgrowth endothelial progenitor cells. Microvasc Res. 2010;79:174. doi: 10.1016/j.mvr.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Fussell G. Cooper S. Synthesis and characterization of acrylic terpolymers with RGD peptides for biomedical applications. Biomaterials. 2004;25:2971. doi: 10.1016/j.biomaterials.2003.09.062. [DOI] [PubMed] [Google Scholar]
- 20.Fussell G. Cooper S. Endothelial cell adhesion on RGD-containing methacrylic terpolymers. J Biomed Mat Res Part A. 2004;70:265. doi: 10.1002/jbm.a.30074. [DOI] [PubMed] [Google Scholar]
- 21.Veleva A. Kahn S. Cooper S. Oxidative and hydrolytic stability of a novel acrylic terpolymer for biomedical applications. J Biomed Mat Res Part A. 2005;74:117. doi: 10.1002/jbm.a.30349. [DOI] [PubMed] [Google Scholar]
- 22.Veleva A. Heath D. Cooper S. Patterson C. Selective endothelial cell attachment to peptide-modified terpolymers. Biomaterials. 2008;29:3656. doi: 10.1016/j.biomaterials.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 23.Veleva A. Heath D. Johnson J. Nam J. Patterson C. Lannutti J. Cooper S. Interactions between endothelial cells and electrospun terpolymer fibers for engineered vascular replacements. J Biomed Mat Res Part A. 2009;91:1131. doi: 10.1002/jbm.a.32276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heath D. Cooper S. Interaction of endothelial cells with methacrylic terpolymer biomaterials. J Biomed Mat Res Part B. 2010;92:289. doi: 10.1002/jbm.b.31514. [DOI] [PubMed] [Google Scholar]
- 25.Heath D. Lannutti J. Cooper S. Electrospun scaffold topography affects endothelial cell proliferation, metabolic activity, and morphology. J Biomed Mat Res Part A. 2010;94:1195. doi: 10.1002/jbm.a.32802. [DOI] [PubMed] [Google Scholar]
- 26.Heath D. Cooper S. Design and characterization of PEGylated terpolymer biomaterials. J Biomed Mat Res Part A. 2010;94:1294. doi: 10.1002/jbm.a.32811. [DOI] [PubMed] [Google Scholar]
- 27.Wang X. Heath D. Cooper S. Endothelial adhesion and proliferation to PEGylated polymers with covalently linked RGD peptides. J Biomed Mat Res Part A. 2012;100(3):794. doi: 10.1002/jbm.a.34026. [DOI] [PubMed] [Google Scholar]
- 28.Nam Y. Park T. Porous biodegradable polymeric tissue scaffolds prepared by thermally induced phase separation. J Biomed Mat Res Part A. 1999;47:8. doi: 10.1002/(sici)1097-4636(199910)47:1<8::aid-jbm2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 29.Lee S. Kim B. Kim S. Choi S. Jeong S. Kwon I. Kang S. Nikolvski J. Mooney D. Han Y. Kim Y. Elastic biodegradable poly(glycolide-co-caprolactone) scaffold for tissue engineering. J Biomed Mat Res Part A. 2003;66:29. doi: 10.1002/jbm.a.10497. [DOI] [PubMed] [Google Scholar]
- 30.Kwon I. Kidoaki S. Matsuds T. Electrospun nano- to microfiber fabrics made of biodegradable copolyesters: structural characteristics, mechanical properties and cell adhesion potential. Biomaterials. 2005;26:3929. doi: 10.1016/j.biomaterials.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Rasband W.S. Image J. U.S. National Institutes of Health, Bethesda; Maryland, USA: 1997–2011. [Google Scholar]
- 32.Salic A. Mitchison T.J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008;105:2415. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Billmeyer F. Textbook of Polymer Science. second. New York: Wiley; 1984. [Google Scholar]
- 34.Painter P. Coleman M. Essentials of Polymer Science and Engineering. Lancaster: DEStech Publications; 2008. [Google Scholar]
- 35.Kokubo T. Kim H. Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials. 2003;24:2161. doi: 10.1016/s0142-9612(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 36.Waite J. Lichtenegger H. Stuckey G. Hansma P. Exploring molecular and mechanical gradients in structural bioscaffolds. Biochemistry. 2004;43:7653. doi: 10.1021/bi049380h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dietz H. Vanaillie P. Svehla M. Walsh W. Steensma A. Vancaillie T. Mechanical properties of urogynecological implant materials. Intl Urogyn J. 2003;14:239. doi: 10.1007/s00192-003-1041-8. [DOI] [PubMed] [Google Scholar]
- 38.Pham Q. Sharma U. Mikos A. Electrospun poly(ɛ-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7:2796. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 39.Megelski S. Stephens J. Chase B. Rabolt J. Micro- and nanostructured surface morphology on electrospun polymer fibers. Macromolecules. 2002;35:8456. [Google Scholar]
- 40.Qian Y. Su Y. Li X. Wang H. He C. Electrospinning of polymethyl methacrylate nanofibres in different solvents. Iran Polym J. 2010;19:123. [Google Scholar]
- 41.Zhao L. He C. Gao Y. Cen L. Cui L. Cao Y. Preparation and cytocompatibility of PLGA scaffolds with controllable fiber morphology and diameter using electrospinning method. JBMR. 2008;87B:26. doi: 10.1002/jbm.b.31060. [DOI] [PubMed] [Google Scholar]
- 42.Deitzel J. Kleinmeyer J. Hirvonen J. Tan N. Controlled deposition of electrospun poly(ethylene oxide) fibers. Polymer. 2001;42:8163. [Google Scholar]
- 43.Kim Y. Cho D. Park W. Electrospinning of poly(dimethyl siloxane) by sol-gel method. J Appl Polym Sci. 2009;114:3870. [Google Scholar]
- 44.Brandrup J. Immergut E. Grulke E. Abe A. Bloch D. Polymer Handbook. fourth. Marblehead, MA: John Wiley & Sons; 2005. [Google Scholar]
- 45.Palacio M. Schricker S. Bhushan B. Block copolymer arrangement and composition effects on protein conformation using atomic force microscope-based antigen-antibody adhesion. JBMR. 2012;100A:978. doi: 10.1002/jbm.a.34033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palacio M. Schricker S. Bhushan B. Bioadhesion of various proteins on random, diblock and triblock copolymer surfaces and the effect of pH conditions. J R Soc Interface. 2011;8:630. doi: 10.1098/rsif.2010.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Angelos M. Brown M. Satterwhite L. Levering V. Shaked N. Truskey G. Dynamic adhesion of umbilical cord blood endothelial progenitor cells under laminar shear stress. Biophys J. 2010;95:3545. doi: 10.1016/j.bpj.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carion A. Domenech J. Hérault O. Benboubker L. Clément N. Bernard M. Desbois I. Colombat P. Binet C. Decreased stroma adhesion capacity of CD34+ progenitor cells from mobilized peripheral blood is not lineage- or stage-specific and is associated with low beta 1 and beta 2 integrin expression. J Hematother Stem Cell Res. 2002;11:491. doi: 10.1089/15258160260090951. [DOI] [PubMed] [Google Scholar]
- 49.Medina R. O'Neill C. Sweeney M. Guduric-Fuchs J. Gardiner T. Simpson D. Stitt A. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genome. 2010;3:18. doi: 10.1186/1755-8794-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vracko R. Benditt E. Capillary basal lamina thickening: its relationship to endothelial cell death and replacement. J Cell Biol. 1970;47:281. doi: 10.1083/jcb.47.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viggers R. Wechezak A. Sauvage L. An apparatus to study the response of cultured endothelium to shear stress. J Biomech Eng. 1986;108:332. doi: 10.1115/1.3138624. [DOI] [PubMed] [Google Scholar]
- 52.Levesque M. Liepsch D. Moravec S. Nerem R. Correlation of endothelial cell shape and wall shear stress in a stenosed dog aorta. Arteriosclerosis. 1986;6:220. doi: 10.1161/01.atv.6.2.220. [DOI] [PubMed] [Google Scholar]
- 53.Inoguchi H. Tanaka T. Maehara Y. Matsuda T. The effect of gradually graded shear stress on the morphological integrity of HUVEC-seeded compliant small diameter vascular graft. Biomaterials. 2007;28:486. doi: 10.1016/j.biomaterials.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 54.Shadwick R. Mechanical design in arteries. J Exp Biol. 1999;202:3305. doi: 10.1242/jeb.202.23.3305. [DOI] [PubMed] [Google Scholar]
- 55.Aird W. Endothelial Biomedicine. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 56.Simionescu N. Simionescu M. Endothelial Cell Dysfunction. New York: Spring Publishing; 1992. [Google Scholar]
- 57.Augustin-Voss H. Smith C. Lewis R. Phenotypic characterization of normal and neoplastic canine endothelial cells by lectin histochemistry. Vet Pathol. 1990;27:103. doi: 10.1177/030098589002700205. [DOI] [PubMed] [Google Scholar]