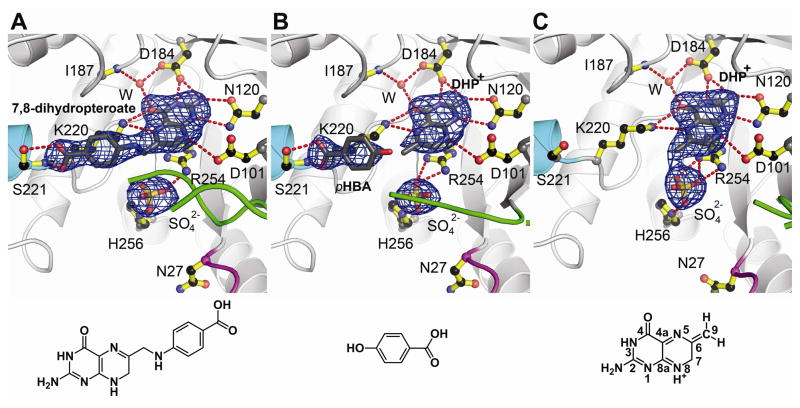
Fig. 1.
Products generated by crystalline BaDHPS. A. The product 7,8-dihydropteroate after soaking crystals in DHPP, pABA and Mg2+. B. DHP+ and pHBA after soaking crystals in DHPP, pHBA and Mg2+. C. DHP+ after soaking crystals in DHPP and Mg2+. In each figure, loop1 is shown in magenta, loop2 is shown in green, the N-terminus of helix αLoop7 is shown in teal, and a sulfate ion occupies the anion binding pocket. The Fo-Fc electron densities were generated from refined structures in which the indicated ligands were omitted, and are contoured at 3σ. Below each figure is the structure of the key molecule bound in the complex. DHP+ is bound in both B and C, but the structure is only shown in C with the pterin ring atoms numbered.