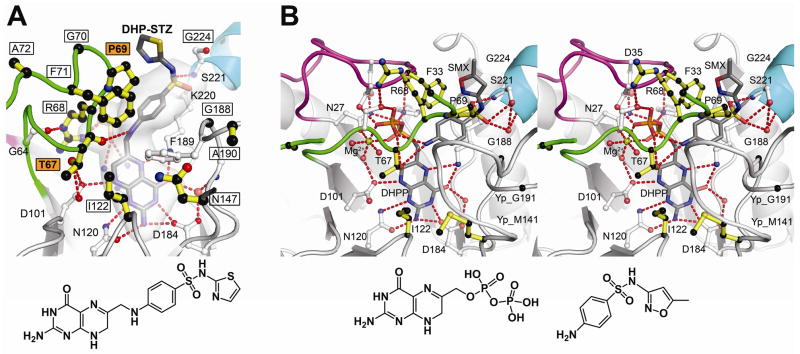
Fig. 3.
Sulfa drug mechanism and resistance. A. B. anthracis (Ba) DHPS crystals soaked in DHPP and sulfathiazole (STZ) reveal a covalent DHP-STZ adduct bound at the active site and stabilized by an ordered loop2. The boxed labeled residues are sites of sulfa drug resistance, and two major sites are labeled in boxed orange. The molecular envelope (light grey) encompasses the pterin- pABA- and anion-binding pockets. Note that the thiazole ring of STZ extends outside this pocket directly adjacent to Pro69. The structure of DHP-STZ is shown below the figure. B. Stereoview of the Y. pestis (Yp) DHPS active site occupied by DHPP, sulfamethoxazole (SMX) and an octahedrally coordinated Mg2+ ion. The structure is very similar to that shown in Fig. 2A that contains pABA in place of SMX. One difference from the pABA structure is that DHPP is intact with the pyrophosphate covalently attached. The residue numbering corresponds to BaDHPS. The structures of DHPP and SMX are shown below the figure. In both figures, loop1 is shown in magenta, loop2 is shown in green, and the N-terminus of helix α Loop7 is shown in teal.