Table 2.
Effect of the substitution and branching at the benzylic positiona
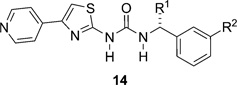 |
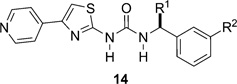 |
||||||
|---|---|---|---|---|---|---|---|
| R1 | R2 | Method, yield | IC50 ± SD (µM) | Method, yield | IC50 ± SD (µM) | ||
| Me | H | (R)-14a | e, 67% | ROCK1 0.043 ± 0.007, (6) | (S)-14a | e, 55% | ROCK1 2.9 ± 0.4, (6) |
| ROCK2 0.012 ± 0.002, (6) | ROCK2 1.9 ± 0.5, (6) | ||||||
| CH2OH | H | (S)-14b | e, 55% | ROCK1 0.03 ± 0.018, (16) | (R)-14b | e, 39% | ROCK1 5.2 ± 0.6, (12) |
| ROCK2 0.01 ± 0.01, (15) | ROCK2 2.5 ± 1, (12) | ||||||
| Eta | H | (R)-14c | e, 37% | ROCK1 0.09 ± 0.012, (12) | (S)-14c | e, 65% | ROCK1 52.2 ± 3.6, (6) |
| ROCK2 0.03 ± 0.01, (12) | ROCK2 22.0 ± 7.8, (6) | ||||||
| CONH2 | H | (S)-14d | f, 30% | ROCK1 0.08 ± 0.01, (12) | (R)-14d | j, 55% | ROCK1 3.7 ± 0.5, (6) |
| ROCK2 0.042 ± 0.016, (12) | ROCK2 2.1 ± 0.36, (6) | ||||||
| CH2OMe | H | (S)-14e | e, 70% | ROCK1 0.07 ± 0.005, (12) | (R)-14e | e, 59% | ROCK1 29.5 ± 3.3, (6) |
| ROCK2 0.03 ± 0.009, (12) | ROCK2 12.0 ± 1.2, (6) | ||||||
| Me | OMe | (R)-14f | e, 50% | ROCK1 0.030 ± 0.022, (15) | (S)-14f | e, 54% | ROCK1 18.7 ± 2.1, (9) |
| ROCK2 0.009 ± 0.0075, (15) | ROCK2 9.3 ± 1.0, (9) | ||||||
| Meb | F | (R)-14g | e, 83% | ROCK1 0.12 ± 0.03, (6) | (S)-14g | e, 78% | ROCK1 30.0 ± 10.0, (4) |
| ROCK2 0.042 ± 0.016, (6) | ROCK2 6.3 ± 0.3, (2) | ||||||
| Me | Cl | (R)-14h | e, 65% | ROCK1 0.64 ± 0.25, (6) | (S)-14h | e, 40% | ROCK1 >50 |
| ROCK2 0.078 ± 0.014, (6) | ROCK2 ND | ||||||
Key: number of repeats shown in parentheses;
(±)-14b, ROCK1 IC50 0.06 ± 0.02 µM (n = 12) and ROCK2 IC50 0.03 ± 0.02 µM (prepared by e, 27%);
(±)-14g, ROCK1 IC50 0.43 ± 0.13 µM (n = 6) and ROCK2 ND (prepared by method e, 83%);
ND = not determined.
