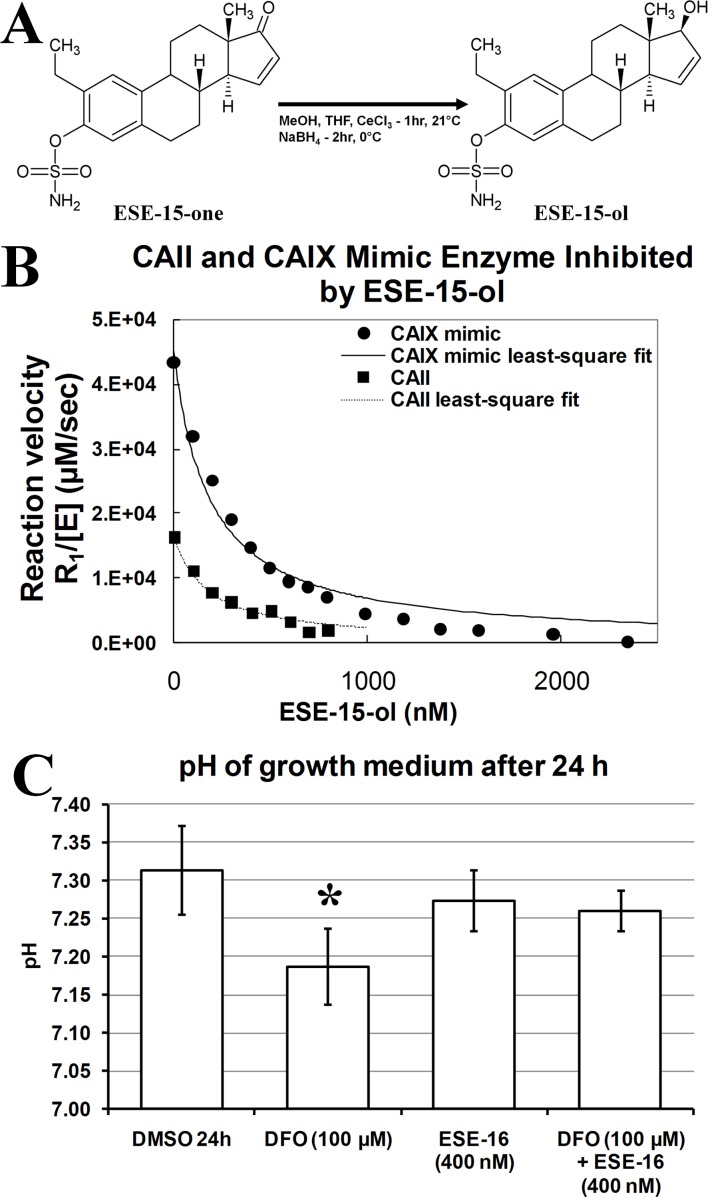
Figure 1. Synthesis of ESE-15-ol, inhibition of carbonic anhydrase activity by ESE-15-ol and the effects of ESE-15-ol on extracellular acidity.
A) Synthesis of 2-ethyl-3-O-sulphamoyl-estra-1,3,5(10),15-tetraen-17-ol from 2-ethyl-3-O-sulphamoyl-estra-1,3,5(10),15-tetraen- 17-one. Reagents and conditions: (a) 2-ethyl-3-O-sulphamoyl-estra-1,3,5(10),15-tetraen-3-ol-17-one (0.11 mmol) and CeCl3 (0.12 mmol) in MeOH :THF (9∶2 v/v) at ambient temperature for 1hr. (b) After 1 hr, reaction was cooled to 0°C and NaBH4 (0.21 mmol) was added and stirred for 2 hr. B) Reaction velocity (R1/[E]) of wild-type CAII and a mimic of CAIX as determined by the catalysis of 18O exchange. Wild-type CAII Ki = 167±19 nM and CAIX mimic Ki = 89±23 nM, calculated using the Henderson method for tight-binding inhibitors. C) Changes in extracellular pH of confluent MDA-MB-231 cells treated with the CAIX inducer, DFO, and ESE-15-ol and a combination of DFO and ESE-15-ol. ESE-15-ol inhibited DFO-induced reduction in extracellular pH. * indicates a t-test P-value <0.05 for difference between vehicle-treated control and the DFO-treated samples.