Figure 2. Sw-APP transfected SH-SY5Y cells secrete Aβ oligomers and had increased intracellular Aβ oligomers level.
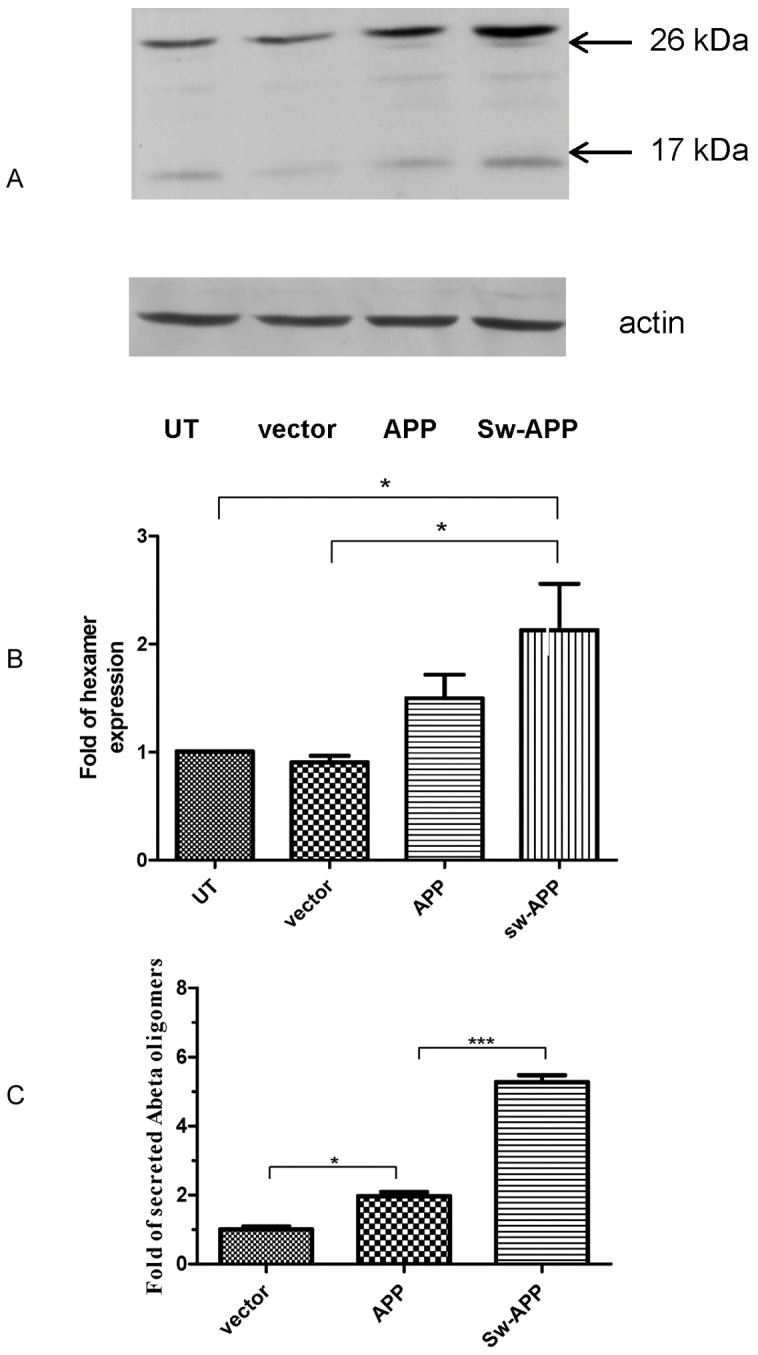
A. Western blot analysis of cell lysates with Aβ antibody. Immunoblotting demonstrated that SH-SY5Y cells transfected with Swedish amyloid precursor protein (Sw-APP) mutant had increased amount of intracellular Aβ oligomers (tetramers and hexamers) compared to SH-SY5Y cells transfected with wild-type amyloid precursor protein (APP), empty-vector (vector) or untransfected SH-SY5Y cells (UT). B. Image J quantitiative analysis confirmed that Sw-APP transfected cells (Sw-APP) had increased amount of intracellular hexamers compared to empty-vector transfected (vector) (p<0.05) or untransfected (UT) cells (p<0.05). C. ELISA for concentration of secreted Aβ oligomers in cultured medium. Sw-APP transfected SH-SY5Y cells (Sw-APP) secreted greater amount of extracellular Aβ oligomers than wt-APP transfected cells (APP) (p = 0.0001), which also secreted greater amount of extracellular Aβ oligomers than empty-vector transfected cells (vector) (p<0.05). Results shown were mean values from 3 independent cultures, and compared by one-way ANOVA using Newman-Keuls multiple comparison test.
