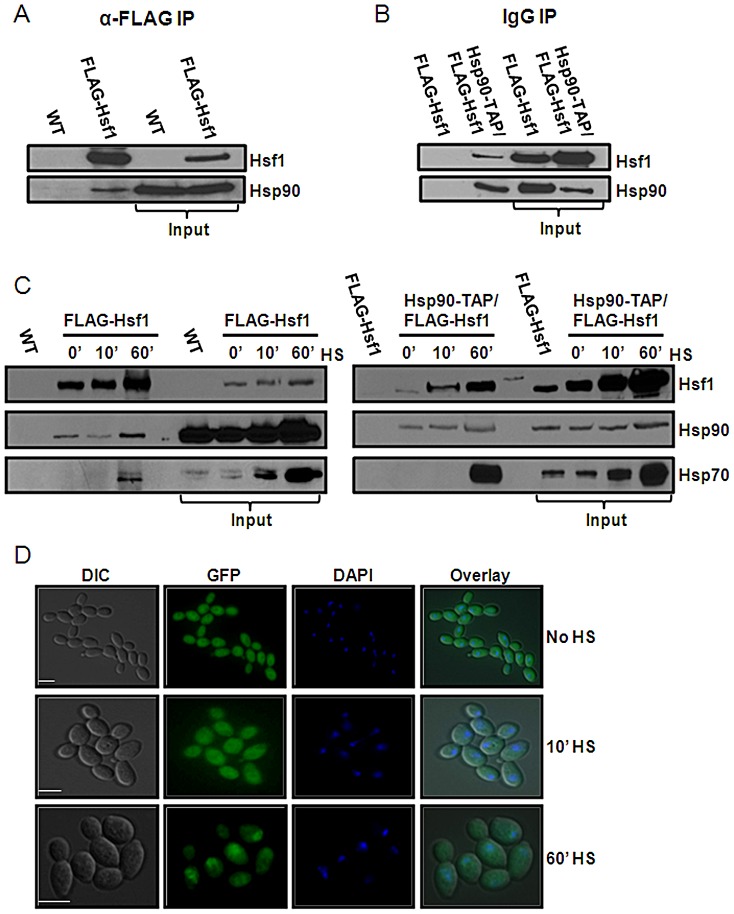
Figure 2. Hsf1 is an Hsp90 client in C. albicans.
Hsf1 and Hsp90 physically interact as revealed by co-immunoprecipitation. (A) Hsp90 co-purifies with FLAG-Hsf1 after immunoprecipitation of extracts from unstressed cells with anti-FLAG M2 affinity agarose. Hsp90 was not co-immunoprecipitated from control cells lacking tagged Hsf1. (B) Reciprocal co-immunoprecipitation was performed using Hsp90-TAP showing that FLAG-Hsf1 copurifies with Hsp90-TAP upon immunoprecipitation with IgG agarose. FLAG-Hsf1 was not co-immunoprecipiated with IgG agarose in control cells lacking FLAG-Hsf1. Data reflect the outcomes for three independent replicate experiments. (C) Hsp90 co-immunoprecipitates with FLAG-Hsf1 0, 10 and 60 minutes after a 30°C to 42°C heat shock, but does not co-immunoprecipitate in control cells lacking FLAG-Hsf1. The membrane was re-probed for Hsp70, which co-purifies with FLAG-Hsf1 at 60 minutes post-heat shock. The reciprocal co-immunopreciptation validates these results: FLAG-Hsf1 co-immunoprecipitates with Hsp90-TAP at 0, 10 and 60 minutes post-heat shock. Re-probing the membranes for Hsp70, reveals that Hsp70 interacts with Hsp90-TAP at 60 minutes post-heat shock. (D) Localisation of Hsp90-GFP in response to elevated temperature. C. albicans CaLC1855 cells were treated with a 42°C heat shock and fixed at 0, 10 and 60 minutes post-heat shock. Hsp90 is localised in the cytosol at 0 and 10 minutes post-heat shock. Significant accumulation of Hsp90 in the nucleus is observed 60 minutes post-heat shock, as determined by co-staining with DAPI. Scale bars, 5 µm.