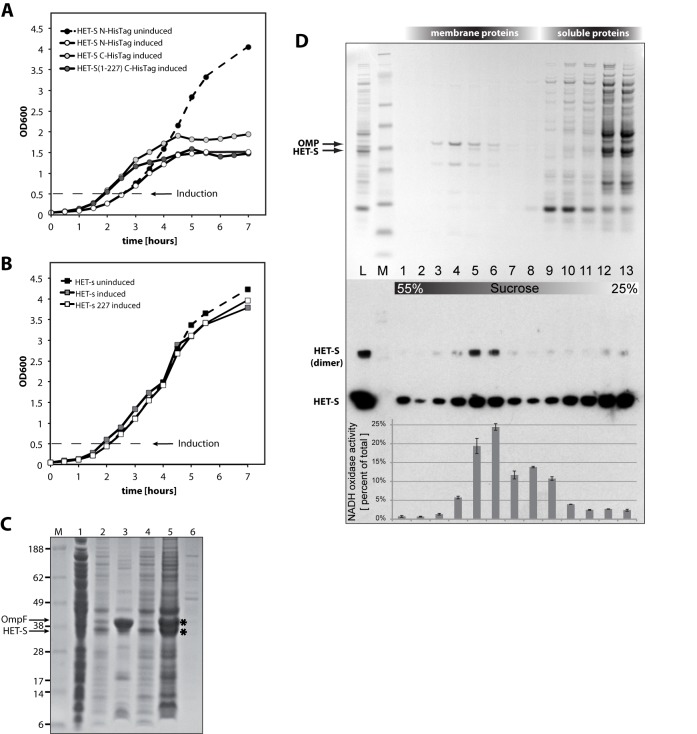
Figure 2. HET-S but not HET-s expression is toxic in E. coli.
The growth of E. coli cultures expressing various constructs of (A) HET-S (full-length, 1–227, or N-terminal Histag) or (B) HET-s (full-length, 1–227) was monitored by their OD600. After induction of protein expression, the cell growth of cultures with HET-S or HET-S(1–227) but not HET-s or HET-s(1–227) was suppressed, indicating that the HET-S HeLo domain, but not HET-s or its HeLo domain, is toxic to E. coli. Further analysis of the cell pellet showed that HET-S is associated with the inner membrane of E. coli. (C) SDS-PAGE analysis of soluble and insoluble fractions after expression in E. coli at 37°C for 4 h. Lanes 1 and 2 represent the supernatant and pellet of the cell lysate, respectively. The pellet and supernatant fractions after treatment with 1 M urea are in lanes 5 and 6, respectively. HET-S remains in the insoluble fraction after incubation in 1 M Urea (lane 5).The pellet and supernatant fractions after extraction with the detergent N-lauroylsarcosine are shown in lanes 3 and 4, respectively, showing that HET-S is solubilized by this detergent. The identity of the HET-S and OmpF bands were confirmed by trypsin digest followed by MS analysis and are marked with an asterisk. (D) Coomassie stain (top) and Western blot (bottom) visualization of SDS-PAGE fractions from a sucrose density gradient separation of E. coli cell lysates after HET-S expression at 37°C for 4 h (lanes 1–13). Lanes L and M are the cell lysate before density gradient separation and the protein marker (SeeBlue Plus2, Invitrogen), respectively. The relative NADH oxidase activity of each fraction (indicating the location of the inner membrane) is plotted over the Western blot. HET-S is found in fractions that correspond to the inner membrane, whereas the outer membrane protein OmpF moves to a higher density. The majority of HET-S is in the soluble protein fractions while a small amount is also found on the very bottom of the tube (55% sucrose, fraction 1) whose density (>1.25 g/ml) indicates that it likely to be lipid-free (or low-lipid-content) protein aggregates.