Abstract
Purpose
To evaluate macular and extramacular retinal anatomy in patients and carriers of X-linked retinoschisis (XLRS) utilizing a wide-field spectral-domain optical coherence tomography (SD-OCT) imaging technique.
Design
Case series
Participants
Six XLRS affected males and three XLRS female carriers.
Methods
The subjects prospectively underwent XLRS DNA genotyping and comprehensive ophthalmic examination including Visual acuity, 30-2 Humphrey visual field, fundus photography, and wide-field SD-OCT, a montage technique to generate SD-OCT images spanning approximately 50° horizontally and 35° vertically of the posterior pole.
Main Outcome Measures
distribution and location of schisis cavities
Results
Among affected XLRS males, asymmetric bilateral schisis was seen in all eyes imaged with montage SD-OCT (11 eyes). Wide-field OCT images demonstrated schisis cavities only in the central macula in 6 eyes (55%), throughout the macula extending to the outside of the temporal arcades in 3 eyes (27%), and throughout the macula extending nasal to the optic nerve in 2 eyes (18%). Cystoid spaces accounting for macular splitting were present in the inner nuclear layer (INL) in all 11 eyes and also in the outer nuclear layer (ONL) in 4 eyes. A few small cysts were seen parafoveally in the ganglion cell layer and/or nerve fiber layer (GCL/NFL) in 4 eyes. Subclinical extramacular schisis spaces were seen (n=5 eyes) within the INL in 1 eye, ONL in 1 eye, INL/GCL/NFL in 1 eye, ONL/GCL/NFL in 1 eye, and in the INL/ONL/GCL/NFL in 1 eye. Schisis was rarely seen nasal to the optic nerve (2 eyes). Central/paracentral visual field defects were seen in 9 eyes. Female carriers did not show schisis on exam or OCT.
Conclusions
Wide-field SD-OCT is a useful tool for evaluating complex retinal anatomy. In XLRS patients, the foveomacular schisis was seen most frequently in the INL. Subclinical extramacular schisis was seen in 45% eyes and was equally prevalent in the INL, ONL, and GCL/FNL. GCL/FNL cystoid spaces were very small and were seen near the fovea and the arcades only. Carriers were schisis-free.
X-linked retinoschisis (XLRS) is a bilateral, recessively inherited vitreoretinal degeneration characterized clinically by stellate spoke-like foveal schisis in up to 100% of affected eyes and peripheral retinoschisis in about half of the affected eyes. 1 Retinal anatomy in this disorder has been a subject of interest and controversy since the 1960’s, beginning with publication of two histopathologic reports describing the splitting of the retinal nerve fiber layer (NFL) in enucleation specimens with advanced disease complicated by retinal detachment. 2,3 Foveal schisis was not described in these early reports. In the era of the optic coherence tomography (OCT), live retinal anatomy is being described, and many publications to date have established that the foveomacular splitting can involve any retinal layer, most frequently the deeper retinal layers, i.e. the inner nuclear (INL) and outer nuclear layers (ONL). 4–13 Some published macular images showed minor cystoid changes in the ganglion cell layer (GCL) and nerve fiber layers (NFL), that were much less prominent than the cysts seen in the deeper layers. 4, 8, 10, 13 While the macular anatomy has been well studied with the OCT, 4–12 the extramacular or peripheral schisis has not been well documented. Interestingly, it has been suggested that schisis seen at the temporal retinal vascular arcades might be more superficial, i.e. near the retina NFL. 11, 12 This raised a question of whether different retinal layers split in different fundus locations. Could it be possible that schisis becomes more superficial further away from the fovea? This would explain the different findings in macular schisis documented by OCT and extramacular schisis documented in the original enucleation specimens.
The goal of the present work was to employ a novel montage technique for spectral-domain OCT (SD-OCT) 14 to create wide-field SD-OCT images of the entire posterior pole in patients with X-linked retinoschisis, including the macular and the extramacular regions. We present findings from these wide-field montage OCT datasets spanning approximately 50° horizontally and 35° vertically of the posterior pole in affected XLRS males and female carriers.
METHODS
The current prospective study was approved by the institutional review board (IRB) at the University of Miami Miller School of Medicine and was compliant with the Health Insurance Portability and Accountability Act of 1996. Patients with XLRS were identified from the Bascom Palmer Eye Institute billing system. These patients were invited to participate in this prospective study. Six affected male patients and three female carriers (whose sons were participating) were recruited. Informed consent was obtained from all subjects.
The following were collected: best corrected Snellen and Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity, intraocular pressure, dilated fundus examination, 30-2 SITA standard Humphrey visual field, montage fundus photography, and wide-field spectral-domain OCT images (see below). The XLRS genotypes were determined at the Carver nonprofit genetic testing laboratory at the University of Iowa.
A single Cirrus HD-OCT unit (Carl Zeiss Meditec, Inc. Dublin, CA) was used to acquire all SD-OCT images from both eyes of each subject. The Cirrus HD-OCT uses a superluminescent diode centered at a wavelength of 840 nm and generates an axial resolution of near 5 μm.
A total of 8 partially overlapping SD-OCT images were obtained for each eye from each subject using the fovea and the optic disc as landmarks for orientation. All OCT images were acquired using the 200 × 200 cube scans pattern (the first number refers to the number of A-scans in each horizontal B-scan; the second number is the total number of horizontal B-scans) covering a field of view of 20×20 degrees (approximately 6mm × 6mm on the retina). Our protocol included scans centered on the fovea and on the optic nerve head, four partially overlapping scans “anchored” by including the fovea in one of the scan’s corners, and two overlapping scans of the region nasally to the optic nerve head “anchored” by including the nerve head at a corner of each scan. Multiple acquisitions for each scanning area were obtained as needed. Images with low signal strength and motion artifacts were excluded.
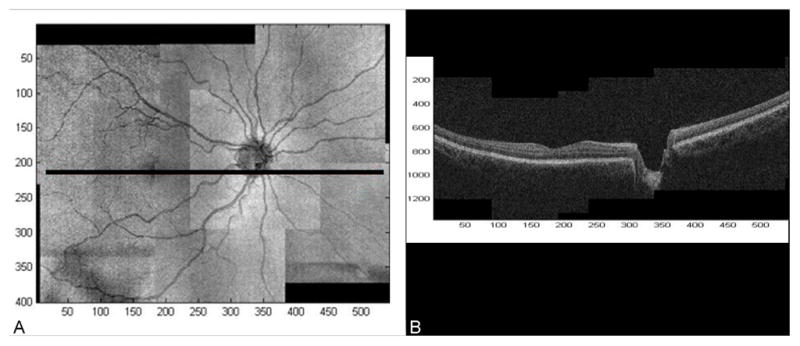
This protocol provided images covering a total region in the posterior pole extending at least 50° horizontally and 35° vertically (approximately 15 mm horizontally and 10.5 mm vertically -- Figure 1A).
Figure 1.
Each SD-OCT raster scan is associated with an OCT fundus image (OFI) obtained by summing the OCT intensities along the A-scans. 15 OFIs are qualitatively similar to traditional en-face retinal imaging modalities (i.e. fundus photography) and make it possible to track precisely the location of each A-scan by its relative position with respect to the visible retinal landmarks. The OFIs can be used to register OCT datasets, both with other OCT images of the same eye and with other en-face retinal imaging modalities (i.e. fundus photography). 16
In addition, partially overlapping SD-OCT datasets can be pieced together to produce a composite 3D OCT image over a large field of view. 14 We used this technique to create an OCT montage from the 8 overlapping OCT datasets acquired using the protocol described above. We refer to the resulting OCT montage as a wide-field OCT (with a corresponding wide-field OFI, Figure 1A). It should be noted that a wide-field OCT is a full 3D dataset containing cross-sectional information over the same wide-field region (Figure 1B).
RESULTS
Six XLRS affected males (11 eyes), age 5 to 49 years, and three XLRS carrier mothers (6 eyes), age 37 to 45 years, completed the study. (Tables 1, 2, available at http://aaojournal.org ). All eyes underwent montage SD-OCT except for the left eye of Patient 2 (5-year-old) where only macular SD-OCT images were obtained due to poor cooperation.
Table 1 (available at http://aaojournal.org) summarizes the findings from the XLRS affected males. Visual acuities ranged from 20/40- to 20/200-. On clinical examination, bilateral cystic maculopathy was present in 5 patients (10 eyes). Bilateral foveal atrophic lesion with lamellar holes were documented in two eyes of an older patient (Patient 3). Wide-field OCTs were constructed in 11 eyes and showed asymmetric bilateral schisis in all patients, in terms of size, density, and location of cystoid spaces. The central macula contained cystoid spaces in all 11 eyes. Three eyes (27%) demonstrated schisis diffusely through the macula to the outside of the arcades. Two eyes (18%) with the most prominent macular schisis also contained small cystoid spaces nasal to the optic nerve. The more anterior nasal periphery was schisis-free.
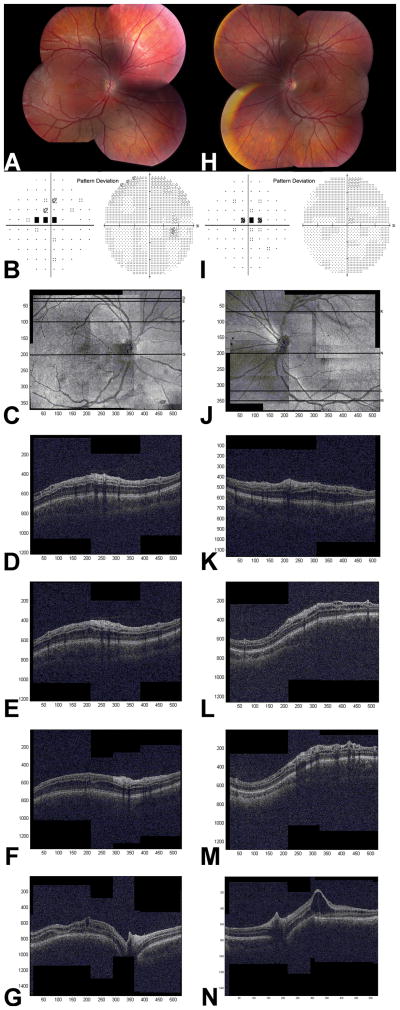
On clinical examination, retina outside of the central macula appeared schisis-free in all eyes; however, wide-field OCT images demonstrated lamellar 10 schisis within the peripheral macula in 5 of 11 eyes (45%) imaged, localized to INL alone in 1 eye and to INL/ONL in 4 eyes. Extramacular schisis was present in 5 eyes (45%), and was located within INL in 1 eye, ONL in 1 eye, INL/GCL/NFL in 1 eye, ONL/GCL/NFL in 1 eye, and in INL/ONL/GCL/NFL in 1 eye. Therefore, extramacular cysts were seen with equal frequency in the INL (3 eyes), ONL (3 eyes), and GCL/NFL (3 eyes). Representative images (Patient 4) are shown in Figure 2.
Figure 2.
Looking at individual retinal layer anatomy, cystoid spaces were present in INL in 11 eyes (100%), ONL in 4 eyes (36%), and GCL/NFL in 4 eyes (36%). The INL schisis was seen either centrally in a circular fashion centered on the fovea or throughout the entire macula reaching the vascular arcades, becoming larger toward the fovea (Figure 2G, N). Cystoid spaces in ONL usually spanned the entire macula horizontally, but the distribution was variable with denser spaces seen in the superior macula and outside of the superior vascular arcades (Figure 2). Minute GCL/NFL cystoid spaces were seen either parafoveally or near the superior and inferior vascular arcades only (Figure 2D, L). The two eyes with nasal schisis contained small cystoid spaces in INL (2 eyes), ONL (1 eye), and very small GCL/NFL spaces supero-nasally to the optic nerve (1 eye).
Far peripheral schisis was present in 8 eyes (67%). These areas were too peripheral to be captured by the OCT. No correlation between the presence of peripheral schisis and OCT appearance or between visual acuity and macular anatomy, other than low vision of 20/200−2 with atrophic macula in Patient 3, was identified.
Central/paracentral visual field defects were seen in 9 eyes. Patient 2 had difficulty completing visual field testing due to young age. His visual fields were not reliable and showed generalized depression in each eye.
Four different genotypes were detected in these 6 patients as listed in Table 1 (available at http://aaojournal.org). Three members of the same family, Patient 1 and 2 (brothers) as well as Patient 3 (uncle), had the same base pair deletion/insertion.
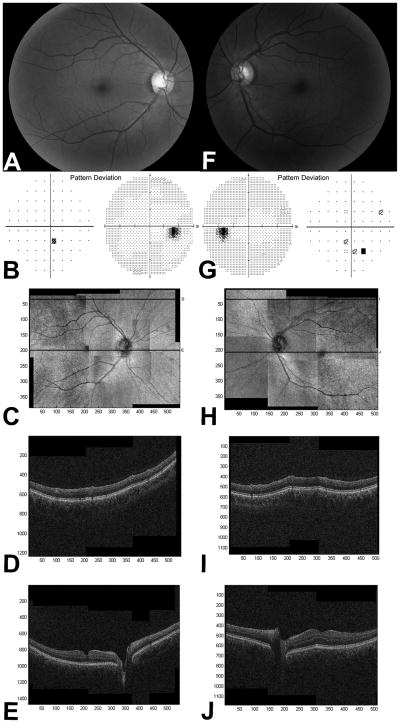
Table 2 (available at http://aaojournal.org) summarizes the findings from the XLRS female carriers. Mothers of Patient 1 and 2, Patient 4, and Patient 5 were included in the study. The fundus examination was unremarkable in two females, while the third showed an abnormally wide foveal reflex (Carrier 3). This subject had a completely normal foveal contour on the OCT in each eye. One eye (Carrier 1) had a slightly irregular foveal contour which was most likely due to an early epiretinal membrane (Figure 3). No macular or extramacular schisis was present in any eyes. The retinal periphery was normal in all eyes on examination. VF showed paracentral defects in each eye of 2 participants (Carrier 1 and 3, Table 2, available at http://aaojournal.org). Genetic analysis revealed heterozygozity for the same mutation found in their corresponding male children.
Figure 3.
DISCUSSION
Using OCT montages we obtained wide-field SD-OCTs covering retinal area of approximately 50° horizontally and 35° vertically to study the retinal anatomy in affected males and female carriers of XLRS. The results of our study are relevant as potential treatments of XLRS are developed, and wide-field SD-OCTs may become a useful outcome measure in future XLRS clinical trials. All affected males exhibited schisis within several retinal layers, which was asymmetric in terms of size, density, and location of cystoid spaces. INL spaces were seen in all eyes: 55% in the central macula only, 18% throughout the macula extending to the outside of the temporal arcades, and another 18% spanning the entire macula to the temporal arcades plus area nasal to the optic nerve. Thirty six percent of affected eyes showed ONL schisis with variable location within the macula and superior to supero-temporal arcade and the optic nerve. Thirty six percent demonstrated multifocal small GCL/NFL spaces located exclusively near the vascular arcades and parafoveally. Female carriers exhibited no schisis.
Montage SD-OCT allowed simultaneous visualization of the macular and extramacular regions, necessary for understanding complex retinal anatomy with either diffuse or multifocal schisis involving multiple retinal layers. The central macula was more susceptible to splitting than the peripheral macula or extramacular retina. As previously documented, central macular schisis was seen in all eyes, with INL being the most commonly split layer. 12, 8 Subclinical peripheral lamellar macular schisis and extramacular schisis each were present in less than half of the eyes.
With the wide-field OCT scans obtained in our study, we confirm that schisis cavities in XLRS males can occur in a number of different layers of the neurosensory retina and the layers involved vary by fundus location. While cystoid spaces in the INL and ONL usually spanned the entire macular or extramacular B-scans reviewed, the minute GCL/NFL cystoid spaces were seen in clusters either parafoveally or near the vascular arcades only. These images corroborated previous observations made by Urrets-Zavalia et al. and Yu et al. who documented splitting of distinctive retinal layers in the central macula and near the vascular arcades. 11,12 Urrets-Zavalia et al. published StratusOCT scans through the fovea, peripheral macula, and the vascular arcades in two brothers with XLRS. 11 Foveal scans demonstrated schisis cavities within the INL, whereas extramacular scans showed retinal splitting under the retinal NFL. Yu et al. studied 34 eyes and reported foveomacular schisis location in the INL in 29 eyes, in the ONL/OPL in 22 eyes, and in the NFL in 4 eyes. Schisis of the INL and ONL/OPL almost always involved the foveal center, but NFL schisis was seen only in the parafoveal area. In addition, the authors published an OCT image taken near the vascular arcade demonstrating schisis under the NFL. 12 In our study, GCL/NFL cysts were seen parafoveally and near the arcades but not within the rest of the macula, therefore individual 6-mm macular B-scans would not show GCL/NFL spaces until the area just outside of the macula is imaged. It appears that the GCL/NFL is much less prone to schisis formation than the deeper layers. Further work is needed to better define extramacular retinoschisis in this disorder.
By immunohistochemistry, Prenner et al. demonstrated that retinoschisin protein is found in all retinal layers, including both plexiform layers and the vicinity of the photoreceptor inner segments. 10 This finding is supported by the work of Takada et al who demonstrated that all retinal neurons express retinoschisin in the mouse retina during development, beginning with the ganglion cells at the first postnatal day and then progressing successively to amacrine, bipolar, and photoreceptor cells. 17 From postnatal day 14, it is strongly expressed in the outer half of the inner nuclear layer and by photoreceptor inner segment. All classes of retinal neurons, except horizontal cell, are shown to be labeled with retinoschisin antibody in adults. 17 This may explain why schisis can occur in multiple retinal layers. The present work provides additional evidence that various fundus locations and individual retina layers have different levels of resilience to schisis formation with the temporal retina and the INL being most susceptible. Biochemical activities attributed to retinoschisin are the binding of β-2-laminin, αβ-crystallin, phospholipid, galactose and Na/K ATPase–SARM1 complex, which is part of cell adhesion and cell-cell interaction. 18
The gene responsible for X-linked retinoschisis was identified within chromosome band Xp22.2. 19 It consists of 6 exons and encodes a 224-aminoacid protein, termed RS1 or retinoschisin. Multiple disease-causing mutations have been identified. 19 Genotype-phenotype correlation has not been previously demonstrable. 20 Several groups have treated murine retinoschisis models successfully using adeno-associated virus (AAV) vectors. 21,22 Retinal transduction with these AAV vectors results in significant levels of retinoschisin protein in all layers of the retina, and improvement of the disease phenotype, including restoration of the normal positive electroretinogram b-wave and a reduction of the cyst-like structures that are characteristic of the disease. The therapeutic effect was durable and persisted throughout the life of the animal. All layers of the retinoschisin knockout (Rs1-KO) mouse retina can be transduced efficiently with AAV vectors administered by simple vitreous injection. 18 Gene therapy in humans is the next logical step in the search for a cure in this disease. Wide-field OCT may become a useful outcome measure for these patients.
As previously documented, female carriers in our study demonstrated normal visual function and no retinal schisis. 1 One eye of one carrier exhibited an irregular foveal contour, which appears to be an early epiretinal membrane formation. One mother demonstrated an abnormal foveal reflex in both eyes, however the OCT failed to demonstrate any abnormalities. VFs of two females showed a few pinpoint paracentral defects, however their significance is not clear since no structural abnormalities to account for these were detected.
In conclusion, wide-field SD-OCT is a useful technique to study large areas of the retinal anatomy in XLRS. Based on montage SD-OCT images in the present study, the foveomacular retinoschisis was located in the deeper layers, most frequently the INL, while the extramacular schisis was equally prevalent in the INL, ONL, and GCL/NFL.
Supplementary Material
Table 1.
Clinical, SD-OCT, visual field and genotype findings in six affected males with XLRS.
| Subject Number | Age (years) | Snellen BCVA (ETDRS letter score) | Macular appearance | Peripheral retinoschisis | Location of cystoid spaces within the macula by SD-OCT | Location of cystoid spaces within the extramacular area by SD-OCT | 30-2 HVF | RS1 Genotype |
|---|---|---|---|---|---|---|---|---|
| Pt 1 (brother of Pt 2) | 9 | 20/80 − (54) OD, 20/63 (60) OS | Stellate spoke- like OU | Shallow inferotemporal schisis OU | OD: within INL in the central macula (larger subfoveally); ONL throughout macula OS: INL in the central macula (larger cysts subfoveally) |
OD: within ONL at the superotemporal arcade only OS: none |
OD: two paracentral pinpoint defects OS: no defects (Reliable OU) |
Asp118 del1gaC ins18GGTGTGC CTGGCTCTCCA hemizygous |
| Pt 2 (Brother of Pt 1) | 5 | 20/80− (54) OD, 20/63-2 (56) OS | Stellate spoke- like OU | No peripheral schisis OU | OD: within INL throughout the macula (larger subfoveally); within ONL in the superior macula to the level of the fovea; few cysts within GCL/NFL parafoveally OS: within INL and ONL in the central macula |
OD: within ONL superior to the macula and the ON, within INL superior to the ON, few within GCL/NFL near the inferotemporal arcade OS: No images due to poor cooperation |
Generalized depression OD>OS, decreased reliability with 25% false positive rate OU | Asp118 del1gaC ins18GGTGTGC CTGGCTCTCCA hemizygous |
| Pt 3 (uncle of Pt 1 and Pt 2) | 47 | 20/200−2 (33) OD, 20/80 (50) OS | OD: atrophy with lamellar macular hole OS: atrophy with early lamellar hole |
Shallow inferotemporal schisis OU | OD: within INL throughout the entire macula, large lamellar macular hole OS: within INL parafoveally, central retinal thinning with irregular foveal contour |
OD: within INL just inferior to the inferotemporal arcade OS: none |
OD: central defect extending from superior to inferior hemifield OS: smaller central defect (reliable OU) |
Asp118 del1gaC ins18GGTGTGC CTGGCTCTCCA hemizygous |
| Pt 4 | 13 | 20/40−2 (66) OD, 20/80−2 (53) OS | Stellate spoke- like OU | Shallow inferotemporal schisis OU | OD: within INL throughout the macula (larger subfoveally); within ONL throughout the macula (larger in the superior macula) OS: within INL throughout the macula (larger subfoveally); within ONL throughout the macula (larger cysts in the superior macula); few within GCL/NFL at the inferotemporal and superotemporal arcade and parafoveally |
OD: within ONL superior to the macula and superior and inferior to the ON; within GCL/NFL near the superotemporal arcade, supero-nasal to the ON, and at the infero-temporal arcade OS: within INL superior to the superotemporal arcade and nasal to the ON; within GCL/NFL superior to the supero-temporal arcade and the ON |
Central defect OU | Arg213Trp CGG>TGG hemizygous |
| Pt 5 | 9 | 20/50−3 (63) OD, 20/63+2 (62) OS | Stellate spoke- like OU | Peripheral schisis OU | OU: within INL in the central macula (larger subfoveally); few tiny spaces within GCL/NFL parafoveally OU | OU: none | Paracentral defects OU (high fixation losses) | Pro203Leu CCG>CTG hemizygous |
| Pt 6 | 49 | 20/80 (51) OD, 20/100 (39) OS | Honeycomb cystic OU | No peripheral schisis OU | OD: within INL in the central macula (larger subfoveally) OS: tiny INL spaces near the fovea |
OU: none | Central scotoma OU (high fixation losses) | Glu6Stop GAA>TAA hemizygous |
BCVA- Best-corrected Visual Acuity, ETDRS- Early Treatment Diabetic Retinopathy Study, OD- right eye, OS- left eye, OU- both eyes, GCL- ganglion cell layer, NFL- retinal nerve fiber layer, INL- inner nuclear layer, ONL- outer nuclear layer, ON-optic nerve, SD-OCT- spectral-domain optical coherence tomography, HVF- Humphrey Visual Field, RS1- retinoschisin gene, Pt- patient, XLRS- X-linked retinoschisis
Table 2.
Clinical, SD-OCT, visual field and genotype findings in three mothers, carriers of XLRS.
| Subject Number | Age (Years) | Snellen BCVA (ETDRS) | Macular appearance | Peripheral retinoschisis | Macular anatomy by SD- OCT | Extramacular midperipheral anatomy by SD-OCT | 30-2 HVF | RS1 Genotype |
|---|---|---|---|---|---|---|---|---|
| Carrier 1 (Mother of Pt 1 and Pt 2) | 43 | 20/20-1 (89) OD 20/20-2 (88) OS | Normal macula OU | Normal periphery OU | OD: slightly irregular foveal contour, no schisis OS: normal, no schisis |
No schisis OU | Single paracentral defect OD, three paracentral defects OS | Heterozygous for the RS1 genotype for patients 1 and 2 in Table 1 |
| Carrier 2 (Mother of Pt 4) | 45 | 20/20+ (86) OD 20/20+ (87) OS | Normal macula OU | Normal periphery OU | OU: normal, no schisis | No schisis OU | No defects OU | Heterozygous for the RS1 genotype for patient 4 in Table 1 |
| Carrier 3 (Mother of Pt 5) | 37 | 20/16 (89) OD 20/20+2 (87) OS | Abnormal macular reflex OU | Normal periphery OU | OU: normal, no schisis | No schisis OU | Two paracentral defects OU | Heterozygous for the RS1 genotype for patient 5 in Table 1 |
BCVA- Best-corrected visual acuity, ETDRS- Early Treatment Diabetic Retinopathy Study, HVF- Humphrey visual field, RS1- Retinoschisin gene, Pt- Patient, SD-OCT- Spectral-domain optical coherence tomography, XLRS- X-linked retinoschisis
Acknowledgments
Supported in part by grant W81XWH-09-1-0674 from the Department of Defense, core grant P30 EY014801 from the National Eye Institute and an unrestricted grant from Research to Prevent Blindness. The sponsor or funding organization had no role in the design or conduct of this research.
Giovanni Gregori, PhD receives research support from Carl Zeiss Meditec.
Footnotes
The data presented in this manuscript was presented at the 2011 Retina Society meeting in Rome, Italy, September 2011 and American Academy of Ophthalmology Annual Meeting in Orlando, FL, October 2011
No conflicting relationships exist for other authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sieving PA, MacDonald IM, Meltzer MR, Smaoui N. Pagon RA, Bird TD, Dolan CR, editors. X-linked juvenile retinoschisis. [Accessed June 7, 2012];GeneReviews [database online] Updated May 12, 2009. Available at: http://www.ncbi.nlm.nih.gov/books/NBK1222/
- 2.Yanoff M, Kertesz Rahn E, Zimmerman LE. Histopathology of juvenile retinoschisis. Arch Ophthalmol. 1968;79:49–53. doi: 10.1001/archopht.1968.03850040051014. [DOI] [PubMed] [Google Scholar]
- 3.Manschot WA. Pathology of hereditary juvenile retinoschisis. Arch Ophthalmol. 1972;88:131–8. doi: 10.1001/archopht.1972.01000030133002. [DOI] [PubMed] [Google Scholar]
- 4.Apushkin MA, Fishman GA, Janowicz MJ. Correlation of optical coherence tomography findings with visual acuity and macular lesions in patients with X-linked retinoschisis. Ophthalmology. 2005;112:495–501. doi: 10.1016/j.ophtha.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Brucker AJ, Spaide RF, Gross N, et al. Optical coherence tomography of X-linked retinoschisis. Retina. 2004;24:151–2. doi: 10.1097/00006982-200402000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson U, Larsson E, Holmstrom G. Optical coherence tomography in the diagnosis of juvenile X-linked retinoschisis. Acta Ophthalmol Scand. 2004;82:218–23. doi: 10.1111/j.1600-0420.2004.00227.x. [DOI] [PubMed] [Google Scholar]
- 7.Gao H, Kusumi R, Yung CW. Optical coherence tomographic findings in X-linked juvenile retinoschisis. Arch Ophthalmol. 2005;123:1006–8. doi: 10.1001/archopht.123.7.1006. [DOI] [PubMed] [Google Scholar]
- 8.Gregori NZ, Berrocal AM, Gregori G, et al. Macular spectral-domain optical coherence tomography in patients with X linked retinoschisis. Br J Ophthalmol. 2009;93:373–8. doi: 10.1136/bjo.2007.136127. [DOI] [PubMed] [Google Scholar]
- 9.Ozdemir H, Karacorlu S, Karacorlu M. Optical coherence tomography findings in familial foveal retinoschisis. Am J Ophthalmol. 2004;137:179–81. doi: 10.1016/s0002-9394(03)00736-0. [DOI] [PubMed] [Google Scholar]
- 10.Prenner JL, Capone A, Jr, Ciaccia S, et al. Congenital X-linked retinoschisis classification system. Retina. 2006;26(suppl):S61–4. doi: 10.1097/01.iae.0000244290.09499.c1. [DOI] [PubMed] [Google Scholar]
- 11.Urrets-Zavalia JA, Venturino JP, Mercado J, Urrets-Zavalia EA. Macular and extramacular optical coherence tomography findings in X-linked retinoschisis. Ophthalmic Surg Lasers Imaging. 2007;38:417–22. doi: 10.3928/15428877-20070901-13. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Ni Y, Keane PA, et al. Foveomacular schisis in juvenile X-linked retinoschisis: an optical coherence tomography study. Am J Ophthalmol. 2010;149:973–8. doi: 10.1016/j.ajo.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 13.Gerth C, Zawadzki RJ, Werner JS, Heon E. Retinal morphological changes of patients with X-linked retinoschisis evaluated by Fourier-domain optical coherence tomography. Arch Ophthalmol. 2008;126:807–11. doi: 10.1001/archopht.126.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Gregori G, Lam BL, Rosenfeld PJ. Automatic montage of SD-OCT data sets. [Accessed June 7, 2012];Opt Express [serial online] 2011 19:26239–48. doi: 10.1364/OE.19.026239. Available at: http://www.opticsinfobase.org/oe/fulltext.cfm?uri=oe-19-27-26239&id=225745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiao S, Knighton R, Huang X, et al. Simultaneous acquisition of sectional and fundus ophthalmic images with spectral-domain optical coherence tomography. [Accessed June 7, 2012];Opt Express [serial online] 2005 13:444–52. doi: 10.1364/opex.13.000444. Available at: http://www.opticsinfobase.org/oe/abstract.cfm?uri=oe-13-2-444. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Gregori G, Knighton RW, et al. Registration of OCT fundus images with color fundus photographs based on blood vessel ridges. [Accessed June 7, 2012];Opt Express [serial online] 2011 19:7–16. doi: 10.1364/OE.19.000007. Available at: http://www.opticsinfobase.org/oe/fulltext.cfm?uri=oe-19-1-7&id=209045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takada Y, Fariss RN, Tanikawa A, et al. A retinal neuronal developmental wave of retinoschisin expression begins in ganglion cells during layer formation. Invest Ophthalmol Vis Sci. 2004;45:3302–12. doi: 10.1167/iovs.04-0156. [DOI] [PubMed] [Google Scholar]
- 18.Park TK, Wu Z, Kjellstrom S, et al. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Ther. 2009;16:916–26. doi: 10.1038/gt.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauer CG, Gehrig A, Warneke-Wittstock R, et al. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat Genet. 1997;17:164–70. doi: 10.1038/ng1097-164. [DOI] [PubMed] [Google Scholar]
- 20.Lesch B, Szabo V, Kanya M, et al. Clinical and genetic findings in Hungarian patients with X-linked juvenile retinoschisis. [Accessed June 7, 2012];Mol Vis [serial online] 2008 14:2321–32. Available at: http://www.molvis.org/molvis/v14/a268/ [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng Y, Takada Y, Kjellstrom S, et al. RS-1 gene delivery to an adult Rs1h knockout mouse model restores ERG b-wave with reversal of the electronegative waveform of X-linked retinoschisis. Invest Ophthalmol Vis Sci. 2004;45:3279–85. doi: 10.1167/iovs.04-0576. [DOI] [PubMed] [Google Scholar]
- 22.Min SH, Molday LL, Seeliger MW, et al. Prolonged recovery of retinal structure/function after gene therapy in an Rs1h-deficient mouse model of x-linked juvenile retinoschisis. Mol Ther. 2005;12:644–51. doi: 10.1016/j.ymthe.2005.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.