Abstract
Rationale
The inability to make profitable long-term decisions has been implicated in several psychiatric disorders. There is emerging evidence to support a role for dopamine (DA) in decision making, but our understanding of the role of noradrenaline (NA) and serotonin (5-HT) in decision making, and of possible interactions between the three monoamines, is limited. Moreover, impulsivity has been associated with aberrant decision making, but the underlying mechanisms are incompletely understood.
Objective
To improve our understanding of the neuropharmacological mechanisms of decision making and impulse control.
Methods
We investigated the effects of amphetamine (0.25-1.0 mg/kg), and selective reuptake inhibitors of DA (GBR12909; 2.5-10 mg/kg), NA (atomoxetine; 0.3-3.0 mg/kg) and 5-HT (citalopram; 0.3-3.0 mg/kg) in a rat gambling task (rGT). Since the rGT allows for detection of impulsive action, i.e., premature responding, we also assessed the relationship between decision making and impulsivity.
Results
In the rGT, rats developed an optimal choice strategy from the first session onwards. Elevation of endogenous DA or NA levels increased and decreased impulsivity, respectively, but did not alter decision making. However, simultaneous blockade of DA and NA disrupted decision making, reflected by a relative decrease in choice for the advantageous choice options. Increasing 5-HT neurotransmission did not affect decision making or impulsivity.
Conclusions
These data suggest important, but complementary or redundant roles of DA and NA neurotransmission in decision making processes based on reward probability and punishment. Moreover, impulse control and decision making in the rGT rely on dissociable mechanisms.
Keywords: Decision making, Impulsivity, Dopamine, Serotonin, Noradrenaline, Rat gambling task
Introduction
Optimal decision making in a changeable and unpredictable environment depends on the ability to evaluate relative risks, costs and benefits associated with different response options to determine appropriate courses of action. The inability to make profitable short- and long-term decisions, leading to suboptimal strategy selection or risk taking behavior, has a major negative impact on daily functioning. Indeed, decision making deficits are a core problem in several psychiatric disorders such as drug addiction, attention-deficit hyperactivity disorder (ADHD), pathological gambling, schizophrenia and Parkinson’s disease (Bark et al. 2005; Bechara 2001; Brand et al. 2004; -2005; Cavedini et al. 2002; Ernst et al. 2003; Johnson et al. 2006; Lawrence et al. 2006; Luman et al. 2008; Malloy-Diniz et al. 2007; Rogers and Robbins 2001; Schutter et al. 2011; Shurman et al. 2005). It is therefore of critical importance to understand the neural mechanisms of decision making, and how they are impaired in psychiatric disorders.
The Iowa Gambling Task (IGT) is a widely used task to assess decision making processes. In this task, participants have to accumulate money, by choosing cards from four decks that vary in both the size and probability of gains and losses (Bechara et al. 1994). Unbeknownst to the participants, the two decks that initially appear most attractive (by producing higher gains), are the least profitable in the long run, since they also produce higher losses. The task reflects various components of real-life decision making as it incorporates the unpredictability of the consequences of a choice, the need to weigh short- and long-term gains and losses, and the necessity to exert behavioral control to maximize gains in the long-term (Bechara et al. 1994). In the present study, a rodent model of decision making was used, i.e., rat gambling task (rGT), in which rats are confronted with multiple response options that differ in the probability and magnitude of rewards and punishments (Zeeb et al. 2009; Zeeb and Winstanley 2011). To maximize the total net gain, rats must learn to avoid the risky response options, which are associated with larger food rewards, but also with longer and more frequent punishment time-outs, similar to the optimal strategy in the IGT. Previous experiments have revealed that the choice in the rGT is guided by the integration of the probability and size of the expected reward with the probability and the magnitude of expected punishment (Zeeb et al. 2009).
Monoaminergic neurotransmission critically regulates executive functions at the level of the frontal cortex (Floresco and Jentsch 2011; Robbins and Arnsten 2009). Dopamine (DA), noradrenaline (NA) and serotonin (5-HT) are therefore important candidates for the modulation of decision making. There is emerging evidence to support a role for DA in decision making. For instance, DA agonist therapy has been associated with impairments in decision making in patients with Parkinson’s disease and restless leg syndrome (Gallagher et al. 2007; Seedat et al. 2000; Weintraub et al. 2006). Impaired decision making has also been reported after acute DA depletion in healthy individuals (Sevy et al. 2006). In the rGT, treatment with amphetamine impaired, whereas the DA D2 receptor antagonist eticlopride improved performance. Treatment with DA D1 or D2 receptor agonists did not alter decision making behavior in the rGT (Zeeb et al. 2009). In a different decision making task, where choice for a large food reward was associated with probabilistic footshock punishment, amphetamine reduced risky decision making (Mitchell et al. 2011; Simon et al. 2009; -2011). This effect of amphetamine was blocked by the DA D2 receptor antagonist eticlopride, and mimicked by the DA D2/D3 receptor agonist bromocriptine (Simon et al. 2011). There is sparse evidence for a role of 5-HT and NA in decision making. Chronic treatment with a mixed 5-HT2A-D2 receptor antagonist was found to improve IGT performance in obsessive-compulsive disorder patients (Cavedini et al. 2002), whereas equivocal results have been obtained regarding the effects of selective 5-HT inhibitors on decision making in pathological gambling (Grant and Potenza 2007, Hollander and Rosen 2000; Van den Brink 2012). In the rGT, performance was slightly impaired after treatment with the 5-HT1A receptor agonist 8-OHDPAT (Zeeb et al. 2009). In heroin addicts, treatment with the alpha-2 adrenoceptor agonist clonidine improved decision making performance (Zhang et al. 2012). However, the selective NA reuptake inhibitor reboxetine did not influence gambling task performance in healthy subjects (O’Carroll and Papps 2003). Thus, although there is evidence to indicate involvement of DA in decision making in the IGT and rGT, our understanding of the role of NA and 5-HT, and possible interactions between the three monoamines, is limited.
In clinical studies, the personality trait of impulsivity has been frequently associated with aberrant decision making. High levels of impulsiveness, i.e., acting without consideration of the possible consequences, were found to be related with lower net scores on the IGT (Franken et al. 2008). Likewise, Zermatten et al. (2005) showed that decision making in the IGT was influenced by the impulsivity-related trait of lack of premeditation. In abstinent regular ecstasy users, elevated impulsivity was combined with impaired decision making in a risky decision making task (Morgan et al. 2006). Although these findings suggest an overlap between the neurobehavioral underpinnings of impulsivity and decision making, other studies have reported that impaired decision making and exaggerated impulsivity can manifest independently (Franken and Muris 2005; Jollant et al. 2005; Kreek et al. 2005; Mavaddat et al. 2000; Van der Plas et al. 2009). The fact that different questionnaires (Barrat Impulsiveness Scale, Behavioral Inhibition System Scale, 17 Impulsiveness Scale) and experimental tasks (stop signal reaction time task, matching familiar figures test) were used to identify impulsivity could contribute to the discrepancies between studies, especially since impulsivity is known to be a heterogeneous construct (Evenden 1999).
In order to improve our understanding of the neuropharmacological mechanisms of decision making, we investigated the effects of treatment with amphetamine, as well as the selective DA, NA and 5-HT reuptake inhibitors GBR12909, atomoxetine, and citalopram on behavior in the rGT (Zeeb et al. 2009; Zeeb and Winstanley 2011). On the basis of previous studies, we hypothesized that rGT performance would be impaired by DA reuptake blockade (using amphetamine or GBR12909; Mitchell et al. 2011; Simon et al. 2009; -2011; Zeeb et al. 2009), whereas blockade of the reuptake of NA (O’Carroll and Papps 2003) or 5-HT (Grant and Potenza 2007, Hollander and Rosen 2000) would be less effective. In a previous study, these monoamine reuptake inhibitors were found to differentially influence two dimensions of impulsive behavior, i.e. impulsive action and impulsive choice (Baarendse and Vanderschuren 2012). The setup of the rGT used here also allows for the detection of impulsive action (i.e., premature responding). Therefore, we assessed the relationship between decision making and impulsivity, by comparing their neuropharmacological modulation by the reuptake inhibitors.
Materials & methods
Subjects
Fourty male Lister Hooded rats (Harlan CPB, Horst, The Netherlands), weighing 250-275 g at the start of the experiments, were housed two per cage under reversed lighting conditions (lights on from 19.00 to 07.00 h). After 2 weeks of habituation to the housing conditions, rats were placed on a restricted diet of 14 g of standard rat chow per day. Body weights were monitored on a weekly basis. Feeding occurred in the rats’ home cages at the end of the experimental day. Water was available ad libitum. All experiments were approved by the Animal Ethics Committee of Utrecht University and were conducted in agreement with Dutch laws (Wet op de Dierproeven, 1996) and European regulations (Guideline 86/609/EEC). Five sessions were scheduled per week (one session per day, Monday-Friday) during the dark phase of the light/dark cycle.
Apparatus
Behavioral testing was conducted in 16 identical operant conditioning chambers (30.5×24×21 cm; Med Associates, St. Albans, VT, USA) each enclosed within a ventilated sound attenuating cabinet (Med Associates Inc, Vermont). The boxes were equipped with a fan to provide ventilation and to mask extraneous noise. Set in the curved wall of each box was an array of five holes. Each nose-poke unit was equipped with an infrared detector and a yellow light-emitting diode stimulus light. Sucrose pellets (45 mg, Formula P; Bio-Serv) could be delivered at the opposite wall via a dispenser. The chamber could be illuminated by a white house light, mounted in the center of the roof. Online control of the apparatus and data collection was performed using MEDPC version 1.17 (Med Associates).
Rat gambling task
Habituation and magazine training Animals were first habituated to the operant chambers over two daily sessions, during which sucrose pellets were placed in the response holes and food magazine. Animals were then trained to make a nose-poke response into an illuminated response hole within 10 s to earn a reward, similar to the training for the five-choice serial reaction time task (5CSRT) (Baarendse and Vanderschuren 2012; Carli et al. 1983; Robbins 2002). The spatial location of the stimulus light varied pseudorandomly between trials across holes 1, 2, 4, and 5. Each session consisted of 100 trials and lasted approximately 30 min. After habituation and magazine training, rats are confronted with four choices differing in the probability and magnitude of rewards and punishments (Zeeb et al. 2009; Zeeb and Winstanley 2011).
rGT In brief, animals were tested once daily in a 30-min session. A trial started with a 5-s inter-trial interval (ITI) followed by illumination of holes 1, 2, 4, and 5 for 10 s. A response in an illuminated hole turned off all stimulus lights, and led to either the delivery of reward, or the start of a time-out ‘punishment’ period. If the trial was rewarded, the appropriate amount of sucrose pellets was immediately delivered into the food tray. If the trial was punished, no reward was delivered and the stimulus light within the chosen hole flashed at 0.5 Hz until the punishing timeout had elapsed. We used an adapted version of the rGT, in which animals were first exposed to ten free choice sample sessions before exposure to the forced- and full free choice sessions. In the free sample sessions, the first two choices for each option were rewarded, after which the reward and punishment contingencies associated with the four response options were introduced. The free sample sessions were followed by a forced-choice version for five sessions before moving on to the full free choice task. In the forced-choice version only one hole was illuminated, to equalize experience of the animals with all of four reward and punishment contingencies, thereby preventing the development of abias toward a particular hole. As in the 5CSRT, premature responses were punished by a 5 s time-out period, signaled by illumination of the house light. A trial was scored as an omission if animals failed to respond within 10 s. The reinforcement schedules were designed such that the optimal strategy was to select the two-pellet option (P2) in terms of reward earned per unit time, associated with a 10 s time-out period that occurs 20% of the time (80% chance of reward). The next best option is P1 (5 s time-out, 90% chance of reward). The two disadvantageous options were both associated with larger immediate gain, i.e., three or four sucrose pellets, but also longer time-out periods (P3: 30 s time-out, 50% chance of reward; P4: 40 s time-out; 40% chance of reward). The hypothetical amount of reward that could be obtained if an option was chosen exclusively per session amounted to the following: P2: 411 pellets, P1: 295 pellets, P3: 135 pellets; and P4: 99 pellets. Therefore, the optimal strategy is to prefer the advantageous options, i.e., P2 and P1, which are associated with smaller, immediate gain, but also less punishment resulting in more reward in the long-term, while avoiding the tempting, yet disadvantageous, large reward options associated with greater loss, i.e., P3 and P4. The spatial locations of the pellet choice options (P1-4) were counterbalanced across subjects. That is, half of the animals was tested in a version of the task in which the order of the response options (in holes 1, 2, 4, and 5, respectively) was P1, P4, P2, P3 and the other half of the animals was tested in a version of the task in which the order of the response options was P4, P1, P3, P2.
The percentage of choices was used to determine preferences for each option, calculated as (number of choices for a particular option/number of total choices made) × 100. Choice scores were also assessed, which were calculated as the sum of the two advantageous options (average percentage choice of P1 and P2) and the sum of the two disadvantageous options (average percentage choice of P3 and P4). In addition, the amount of premature responses and omissions made were calculated.
Drugs
(+)-Amphetamine sulphate was purchased from O.P.G. (Utrecht, The Netherlands). GBR12909 dihydrochloride, atomoxetine hydrochloride and citalopram hydrobromide were purchased from Tocris Bioscience (UK). All drug doses were calculated as salt, dissolved in 0.9% saline (amphetamine, atomoxetine, and citalopram) or sterile water (GBR12909). Drugs were freshly prepared each day before testing and injected intraperitoneally (i.p.) in a volume of 1ml/kg body weight. Injections were given 30 min before behavioral testing commenced, since we have previously demonstrated clear-cut effects on impulsivity of these drugs using this pretreatment interval (Baarendse and Vanderschuren 2012). Drug tests were conducted on Tuesdays and Fridays with baseline training sessions on the other weekdays. Pharmacological challenges began once stable baseline behavior had been established. Before the first test day, all animals had been habituated twice to i.p. saline injections. Drugs were administrated as follows: cohorts of 10 rats received three different doses of one drug including saline according to a Latin square design, followed by the same procedure with a second drug. Each animal received a maximum of two different drugs and the order of drugs was counterbalanced between cohorts of rats (amphetamine-citalopram; GBR12909-atomoxetine; citalopram-GBR12909; atomoxetine-amphetamine). Subsequently, a cohort of 20 rats was used to test the effects of drug combinations, which were administrated according to a Latin square design as well. Due to experimental errors, the data of some rats were excluded from the analysis. Animals were tested drug-free for a minimum of 1 week between drugs and drug combinations to prevent carryover effects.
Data Analysis
All data are presented as means and standard errors of the mean and analyzed using SPSS for Windows, version 15.0. An arcsine transformation was performed before analysis of the percentage of choice to limit the effect of an artificially imposed ceiling (McDonald, 2009). Analysis of choice behavior per quartile of trials (Q1-Q4) or free sampling choice sessions (first 10 sessions) and free choice sessions (session 11-29) were performed using a one-way, repeated-measures analysis of variance (ANOVA) with quartile or session as within-subject factor and choice (two or four levels, advantageous-disadvantageous choices and P1-4, respectively) as between-subject factor.
Data from the pharmacological challenges were analyzed using a two-way, repeated-measures analysis of variance (ANOVA) with choice (two or four levels, advantageous-disadvantageous choices and P1-4, respectively) and drug dose (four levels, vehicle plus three doses of compound) or drug combination (four levels, vehicle-vehicle plus combinations of two monoamine reuptake inhibitors) as within-subject factors. If the outcome of the repeated-measures ANOVA yielded significant effects of dose or dose×choice at p<0.05 level, further post-hoc analysis was performed using one-way ANOVA for comparison between choice options or paired samples t-tests for comparison between drug doses.
Results
Development of choice behavior during rGT acquisition
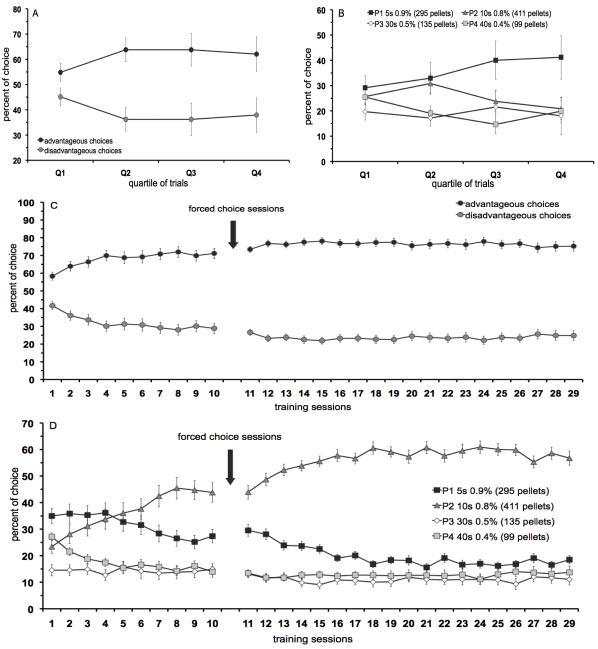
A detailed analysis of choice behavior per quartile of trials during the first free sampling choice session is depicted in figure 1 (A, B). Animals started with an equal preference for the advantageous versus disadvantageous options during the first quartile of trials (Q1), but developed a preference for the advantageous options within the next quartiles of trials (figure 1A) [quartile × choice: F(3,66)=2.70, p<0.05, Q1: NS, Q2-4: p<0.02]. Analysis of the four different pellet options per quartile revealed an effect of choice [quartile × choice: F(9,132)=1.40, NS; choice: F(3,44)=3.36, p<0.03], but post-hoc comparison indicated no significant difference between the pellet options (figure 1B).
Figure 1.
Development of choice pattern in the rGT. Advantageous versus disadvantageous options (A) and four different response options (B) during each quartile of trials within the 1st 30-min session. Advantageous versus disadvantageous options (C) and four different response options (D) over training sessions. In total, n=40 animals were included in the analysis. All data are expressed as mean±SEM. Note that not all scales of the panels are identical.
During subsequent training sessions, there was a significant preference for the advantageous choice options during free sampling choice sessions [session × choice: F[sessions 1-10] (9,702)=10.19, p<0.001] and free choice sessions [session × choice: F[session 11-29](18,1404)=1.96, p<0.01] (figure 1C). Post-hoc analysis indicated a significant difference between the advantageous options versus the disadvantageous options for all training sessions (session 1-29, p<0.001). Analysis per pellet option revealed a significant difference between the four different pellet options during the free sampling choice sessions [session × choice: F[sessions 1-10](27,1404)=10.31, p<0.001] and free choice sessions [session × choice: F[session 11-29](54,2808)=7.33, p<0.01]. Post-hoc analysis indicated that option P1 was significantly different from all other options during sessions 6-7, 10-17, 19 and 22, whereas option P2 was significantly more chosen compared with the other four options from session 7 onwards (p<0.05) (figure 1D).
Effect of amphetamine and monoamine reuptake inhibitors on decision making
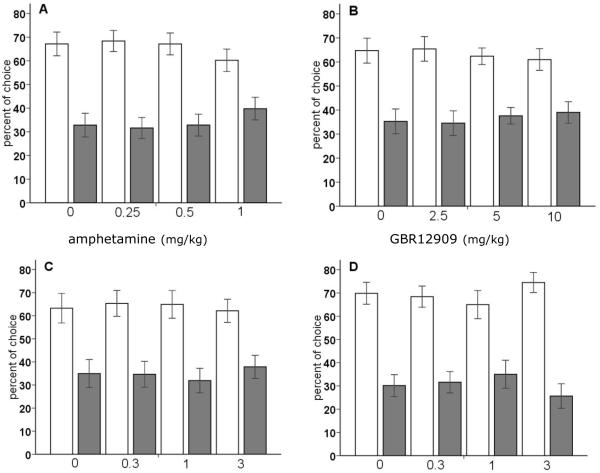
Treatment with neither amphetamine, nor GBR12909, atomoxetine or citalopram significantly influenced choice expressed as percentage of choice for the advantageous versus disadvantageous options (figure 2A-D) [dose × choice: F[amphetamine](3,57) =0.96, NS; F[GBR12909](3,57)=0.28, NS; F[atomoxetine](3,39)=0.35m, NS; F[citalopram](3,54)=1.02, NS]. The effects of treatment with amphetamine and the monoamine reuptake inhibitors on choice behavior expressed per response option, i.e., P1, P2, P3, and P4, are shown in table 1. Administration of amphetamine at a dose of 0.5 mg/kg and 1 mg/kg decreased the choice of P2, and increased choice of P1 [dose × choice: F(9,171)=2.54, p<0.01]. In view of previously reported effects of a higher dose of amphetamine than used here (i.e., 1.5 mg/kg; Zeeb et al. 2009), the effect of 1.5 mg/kg of amphetamine was tested after the dose-effect study with amphetamine. This higher dose of amphetamine significantly shifted choice behavior from the advantageous [saline 67.2%±5; amphetamine 55.9%±4.4] towards disadvantageous options [32.8%±5; amphetamine 44.1%±4.4]. In addition, the pattern of effects on the four response options was comparable to that reported by Zeeb et al. (2009), i.e., an increase in P1 and P4, and a decrease in P2 and P3 (saline: P1: 15±1.5, P2: 52.2±4.7, P3: 17±3.7, 15.8±3.3; amphetamine: P1: 21.3±2.2, P2: 34.6±4.4, P3: 8.2±1.7, P4: 35.8±4.8). DA and 5-HT reuptake inhibition by GBR12909 and citalopram, respectively, did not alter choice behavior for the different options [dose × choice: F[GBR12909](9,171)=1.26, NS; F[citalopram](9,161)=1.06, NS]. The NA reuptake inhibitor, atomoxetine, significantly decreased the choice of option P1 as well as option P4, at an intermediate dose of 1 mg/kg [dose × choice: F(9,117)=1.99, p<0.05] (table 1).
Figure 2.
Effects of amphetamine (A), the selective DA reuptake inhibitor GBR12909 (B), the selective NA reuptake inhibitor atomoxetine (C) and the selective 5-HT reuptake inhibitor citalopram (D) on choice behavior, i.e., advantageous options versus disadvantageous options in the rGT. In total, n=20, n=20, n=14, and n=19 animals were included in the analysis, respectively. Asterisk indicates p<0.05 and two asterisks indicate p<0.01 compared to vehicle treatment (paired samples t-test). All data are expressed as mean±SEM.
Table 1.
Effects of amphetamine, the selective DA reuptake inhibitor GBR12909, the selective NA reuptake inhibitor atomoxetine, the selective 5-HT reuptake inhibitor citalopram and combined administration of the reuptake inhibitors on choice behavior, i.e., percentage of choice for pellet option P1, P2, P3, and P4, and errors of omission in the rGT. In total, n=14-24 animals were included in the analysis. Asterisk indicates p<0.05 and two asterisks indicate p<0.01 compared to vehicle treatment (paired samples t-test). All data are expressed as mean±SEM.
| pellet option | Errors of omission |
||||
|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | ||
|
|
|||||
| Amphetamine | |||||
| Veh | 15.0 ± 1.5 | 52.2 ± 4.7 | 17.0 ± 3.7 | 15.8 ± 3.3 | 9.4 ± 2.2 |
| 0.25 mg/kg | 20.5 ±2.7 | 47.9 ± 4.4 | 10.0 ± 2.1 | 21.6 ± 4.4 | 5.7 ± 1.9 |
| 0.5 mg/kg | 29.2 ± 3.7 ** | 37.9 ± 4.6 * | 12.9 ± 2.2 | 19.9 ± 4.6 | 7.7 ± 2.0 |
| 1.0 mg/kg | 24.6 ± 3.5 * | 35.7 ± 4.1 * | 17.0 ± 4.4 | 22.8 ± 4.1 | 14.2 ± 3.7 |
| GBR12909 | |||||
| Veh | 10.9 ± 1.9 | 53.8 ± 4.3 | 14.4 ± 4.7 | 20.9 ± 3.5 | 6.8 ± 2.3 |
| 2.5 mg/kg | 14.4 ± 2.7 | 51.0 ± 5.1 | 15.5 ± 5.7 | 19.0 ± 3.7 | 7.4 ± 1.8 |
| 5.0 mg/kg | 16.5 ± 2.8 | 46.4 ± 4.6 | 10.7 ± 3.0 | 26.4 ± 3.6 | 3.4 ± 0.8 |
| 10.0 mg/kg | 17.9 ± 3.0 | 43.1 ± 4.9 | 14.9 ± 5.1 | 24.2 ± 3.9 | 8.0 ± 4.5 |
| Atomoxetine | |||||
| Veh | 14.3 ± 1.3 | 49.0 ± 6.7 | 12.8 ± 2.7 | 22.2 ± S.6 | 7.6 ± 2.2 |
| 0.3 mg/kg | 12.4 ± 2.2 | 53.0 ± 5.0 | 12.4 ± 4.0 | 22.2 ± 3.8 | 12.4 ± 2.6 ** |
| 1.0 mg/kg | 10.5 ± 1.8 * | 54.5 ± 5.3 | 17.5 ± 6.0 | 14.4 ± 3.4 * | 21.3 ± 4.5 ** |
| 3.0 mg/kg | 18.4 ± 3.9 | 43.7 ± 4.7 | 10.4 ± 2.1 | 27.5 ± 4.7 | 40.2 ± 8.7 ** |
| Citalopram | |||||
| Veh | 20.3 ± 3.0 | 49.6 ± 4.4 | 14.3 ± 4.1 | 15.9 ± 3.6 | 15.7 ± 4.4 |
| 0.3 mg/kg | 21.2 ± 2.9 | 47.2 ± 4.5 | 12.7 ± 3.7 | 18.9 ± 4.7 | 11.8 ± 3.2 |
| 1.0 mg/kg | 21.2 ± 3.1 | 43.8 ± 5.9 | 20.6 ± 5.2 | 14.4 ± 3.0 | 11.7 ± 3.0 |
| 3.0 mg/kg | 22.0 ± 2.7 | 52.5 ± 3.5 | 12.7 ± 4.2 | 12.8 ± 2.9 | 15.2 ± 3.4 |
| vehicle-vehicle | 10.3 ± 1.7 | 62.2 ± 4.8 | 9.9 ± 2.1 | 17.6 ± 5.0 | 4.5 ± 1.6 |
| GBR12909-atomoxetine | 28.2 ± 3.7 ** | 30.4 ± 3.0 ** | 15.5 ± 1.9 * | 25.9 ± 4.3 ** | 33.3 ± 5.5 ** |
| GBR12909-citalopram | 21.2 ± 3.4 ** | 48.1 ± 4.8 * | 13.4 ± 3.1 | 17.3 ± 3.3 | 5.1 ± 1.7 |
| atomoxetine-citalopram | 26.7 ± 5.3 ** | 42.1 ±4.6 ** | 8.9 ± 1.6 | 22.4 ± 4.9 | 42.4 ± 7.5 ** |
Doses of combined administration of reuptake inhibitors: GBR12909 (10 mg/kg), atomoxetine (3 mg/kg), citalopram (3 mg/kg)
The modest effects of the monoamine reuptake inhibitors lead us to think that there might be interaction between and/or redundancy in the monoamine systems that modulate decision making in the rGT. Therefore, we administered combinations of two monoamine reuptake inhibitors to the animals and evaluated their performance in the rGT. Combined administration of these reuptake inhibitors altered choice behavior in terms of advantageous options versus disadvantageous options, with a significant shift in choice behavior induced by the GBR12909-atomoxetine combination (figure 3) [drug × choice: F(3,57)=4.70, p<0.01]. All combinations of monoaminergic reuptake inhibitors resulted in a significant increase in the choice of P1, which was accompanied by a decrease in choice of P2 [drug × choice: F(9,171)=8.2, p<0.001], comparable with the effect of amphetamine (table 1). However, only with the combination of GBR12909 and atomoxetine this shift in choice behavior was accompanied with significant increase in the choice for P3 and P4, resulting in a shift in advantageous toward disadvantageous choices (p<0.05) (figure 3 and table 1).
Figure 3.
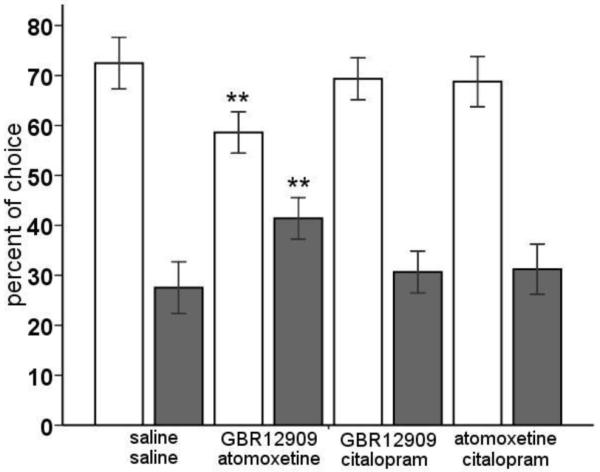
Effects of combined administration of the selective DA reuptake inhibitor GBR12909 (10 mg/kg), the selective NA reuptake inhibitor atomoxetine (3 mg/kg) and/or the selective 5-HT reuptake inhibitor citalopram (3 mg/kg) on choice behavior, i.e., advantageous options versus disadvantageous options in the rGT. In total, n=20 animals were included in the analysis. Asterisk indicates p<0.05 compared to vehicle treatment (paired samples t-test). All data are expressed as mean±SEM.
Effect of amphetamine and monoamine reuptake inhibitors on premature responding and errors of omission
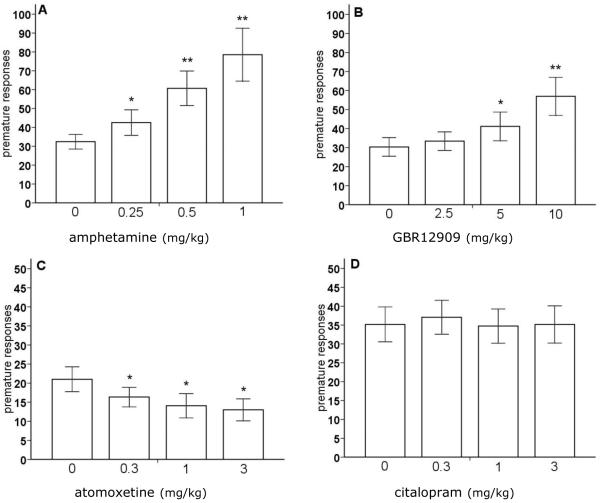
Amphetamine significantly increased the number of premature responses, i.e. responses during the 5 s intertrial interval, in the rGT (figure 4A) [F(3,57)=12.0, p<0.01]. Post-hoc analysis revealed that the increase in premature responses was significant at all doses tested (p<0.05). Systemic administration of GBR12909 resulted in enhanced premature responding at a dose of 5 and 10 mg/kg (figure 4B) [F(3,57)=7.6, p<0.01]. Amphetamine and GBR12909 had no effect on the amount of errors of omission (table 1) [F[amphetamine](3,57)=2.51, NS; F[GBR12909](3,57)=1.23, NS]. On the other hand, atomoxetine decreased premature responses and enhanced the errors of omission at all doses tested (figure 4C and table 1) [F[premature responses](3,39)=4.84, p<0.01, F[errors of omission](3,39)=11.11, p<0.01]. Both premature responding and errors of omission were unaffected by treatment with citalopram (figure 4D and table 1) [F[premature responses](3,54)=0.16, NS; F[errors of omission](3,54)=0.75, NS].
Figure 4.
Effects of amphetamine (A), the selective DA reuptake inhibitor GBR12909 (B), the selective NA reuptake inhibitor atomoxetine (C) and the selective 5-HT reuptake inhibitor citalopram (D) on premature responding, i.e., impulsive action, in the rGT. In total, n=20, n=20, n=14, and n=19 animals were included in the analysis, respectively. Asterisk indicates p<0.05 and two asterisks indicate p<0.01 compared to vehicle treatment (paired samples t-test). All data are expressed as mean±SEM. Note that not all scales of the panels are identical.
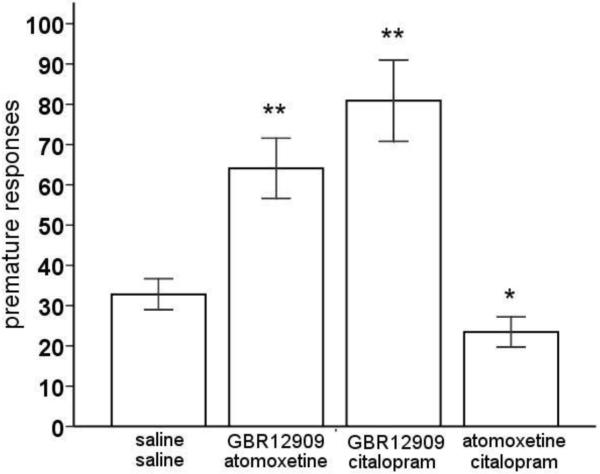
Simultaneous blockade of DA (GBR12909) with either NA (atomoxetine) or 5-HT (citalopram) reuptake significantly increased premature responding in the rGT, whereas blockade of NA (atomoxetine) combined with 5-HT (citalopram) reuptake decreased premature responding (figure 5) [F(3,69)=24.43, p<0.01]. The errors of omission, on the other hand, were increased when DA (GBR12909) or 5-HT (citalopram) reuptake blockade was combined with atomoxetine (table 1) [F(3,69)=20.39, P<0.01], but not when GBR12909 was combined with citalopram.
Figure 5.
Effects of combined administration of the selective DA reuptake inhibitor GBR12909 (10 mg/kg), the selective NA reuptake inhibitor atomoxetine (3 mg/kg) and/or the selective 5-HT reuptake inhibitor citalopram (3 mg/kg) on premature responding, i.e., impulsive action, in the rGT. In total, n=20 animals were included in the analysis. Asterisk indicates p<0.05 and two asterisks indicate p<0.01 compared to vehicle treatment (paired samples t-test). All data are expressed as mean±SEM.
Discussion
In the present study, we used the rGT to show that rats are capable of developing an optimal choice strategy within a limited amount of sessions without knowing the reinforcement contingencies of the different response options. From the first session onwards, rats preferred the advantageous options (P1 and P2) characterized by low pellet gain, but high probability of reinforcement and short punishment time-outs, over the more disadvantageous options (P3 and P4) associated with larger reward size, but lower net gains in the long run. Because the task contingencies were unknown to the rats, the first free choice training session is analogous to gambling behavior in the IGT (Bechara et al. 1994). Similar to healthy human subjects (Bechara et al. 1994; -1999; Brand et al. 2007), the rats displayed a shift from primarily explorative behavior at the beginning of the task, during which they sample all the options, toward a more exploitative strategy involving substantially more choices of the advantageous options. After task acquisition, decision making under known reinforcement schedules was investigated. Simultaneous DA and NA transporter blockade resulted in a shift towards risky decision making reflected by a decrease in choice for the advantageous options and increase in choice for the disadvantageous options. Elevation of endogenous DA or NA levels by single transporter blockade resulted in disrupted impulse control or slowed task performance, respectively, but was not sufficient to disrupt decision making.
Decision making is a complex cognitive function that is guided by the integration of different mental processes, such as the formation of preferences, selection and execution of actions, evaluation of outcomes, sensitivity to loss or punishment, delay aversion, attention and behavioral flexibility (Ernst and Paulus 2005). Monoaminergic neurotransmission may modulate decision making to interfere independently with one or more of these processes. For example, DA has been implicated in incentive motivation, salience of emotionally charged cues or events, and action invigoration (Berridge 2007; Cardinal et al. 2000; Cools et al. 2011; Robbins and Everitt 2007; Salamone et al. 1994; -2007). On the other hand, NA appears to be especially involved in decision making when contingencies are unexpectedly changed and alternatives are explored (Aston-Jones et al. 2005; Bouret and Sara 2005; Cole and Robbins 1992; Dalley et al. 2001; Sirviö et al. 1993; Usher et al. 1999). The contribution of 5-HT to decision making is more complex and is likely to involve multiple receptor systems (for review see, Rogers 2011). Most prominently, 5-HT function has been associated with aversive processing in decision making (Boureau and Dayan 2010; Cools et al. 2008; -2011; Dayan and Huys 2008; Kranz et al. 2010; Tops et al. 2009). The modulatory role of monoaminergic neurotransmission may depend on the complexity of decision making and the processes involved. For example, manipulation of DA neurotransmission has been reported to influence decision making processes based solely on reward or punishment probability (Simon et al. 2011; St Onge and Floresco 2009; Zeeb et al. 2009), whereas the role of DA in decision making appears more limited when reward size, probability and punishment duration are integrated (Zeeb et al. 2009; present study). Together with the data present here, these findings suggest that the neural basis of decision making strongly depends on task complexity and requirement of cognitive effort and conflict resolution (Raylu and Oei 2002).
Elevation of DA, NA or 5-HT by inhibiting transmitter reuptake did not substantially influence choice behavior in the rGT. It is possible that each of these monoamines has a unique role in certain components of decision making, but that these separate roles are not essential for the overall performance of choice behavior in the rGT.The lack of a substantial effect of GBR12909, atomoxetine or citalopram on choice behavior in the rGT can be explained by functional redundancy in monoaminergic function. Indeed, redundant mechanisms have, for example, been described for catecholamine signaling in fear conditioning. Signaling through β2-adrenergic receptors or D5-DA receptors is itself not essential for fear memory consolidation, but if function of these receptors is simultaneously inhibited, through pharmacological or genetic means, consolidation of fear memory is disrupted (Ouyang et al. 2012). In the present study, it was shown that only if DA transporter blockade was combined with NA transporter blockade, decision making was impaired, reflected by a shift from advantageous choices towards more disadvantageous choices. This disruption in choice pattern was specific for the combination of GBR12909 and atomoxetine, as combined administration of GBR12909 or atomoxetine with citalopram did not substantially alter decision making. A detailed analysis of choice for the different options after treatment with GBR12909 and atomoxetine showed that the percentage of choice for P2, the optimal option, was significantly decreased, whereas choice for the advantageous option P1, as well as the risky options P3 and P4 was elevated. Combined DA and NA transporter blockade thereby seems to flatten the choice profile for the different options resulting in impaired decision making. This pronounced flattening of the choice profile, i.e. increased choice for P1 and P4 and decreased choice of P2 and P3, causing a decrease in advantageous choices, was also observed with a higher dose of amphetamine (1.5 mg/kg) (Zeeb et al. 2009; present study). The effect of GBR12909-atomoxetine is not likely the result of a motivational deficit, since increasing DA neurotransmission enhances the willingness of rats to wait longer or work harder for larger rewards in delay and effort discounting tasks (Cardinal et al. 2000; Denk et al. 2005; Floresco et al. 2008a; -2008b; Salamone et al. 1994; Van Gaalen et al. 2006b). Moreover, NA reuptake inhibitors have been shown to increase reinforcement rates in differential reinforcement of low responding schedules (O’Donnell et al. 2005). Since the shift towards the disadvantageous options under GBR12909-atomoxetine was accompanied by an elevation of choice for P1, an increase in the motivation for larger rewards or decreased sensitivity to the punishment delay is also not a plausible explanation of the disruption in decision making. More likely, combined DA and NA reuptake inhibition seems to impair the ability to distinguish between options that are associated with differences in probability of reward and punishment. Catecholamine neurotransmission has been closely implicated in fronto-executive functioning, whereby DA and NA may have complementary functions (for review see, Robbins and Arnsten 2009). Thus, prefrontal DA and NA signaling has to be within a critical range to enable functions such as attentional selection, resistance to interference, monitoring, behavioural inhibition, task switching and planning (Arnsten 1997; -2000; Dalley et al. 2004; Floresco and Magyar 2006; Robbins and Arnsten 2009; Zahrt et al. 1997). Combined disruption of two or more of these component processes by inhibiting the reuptake of DA and NA may then lead to the impairments in decision making observed here, although there may also be functional redundancy in the role of DA and NA in decision making.
An alternative explanation for the disruption in decision making after combined treatment with GBR12909 and atomoxetine is a pharmacokinetic interaction, whereby administration of one drug enhances the bioavailability of the other. Importantly, however, GBR12909 and atomoxetine did not influence each other’s effects on premature responses or errors of omission (see Figure 4, 5 and Table 1). That is, in the presence of GBR12909 (which did not affect errors of omission by itself), the effect of atomoxetine on errors of omission was comparable to that of atomoxetine alone. The same holds true for premature responses: in the presence of atomoxetine, the effect of GBR12909 was comparable to that of GBR12909 alone (in which case the effect of GBR12909 appeared to overshadow the modest reduction in premature responses caused by treatment with atomoxetine). If a pharmacokinetic interaction between GBR12909 and atomoxetine underlies its effects on choice in the rGT, then one might expect this to be reflected in the other task parameters as well. Furthermore, since the combination of GBR12909 and atomoxetine yielded effects on decision making in the rGT that closely resembled those of a high dose of amphetamine, we think that a pharmacokinetic explanation of these data is unlikely.
The personality trait of impulsivity has been frequently associated with impaired decision making (Franken et al. 2008; Hanson et al. 2008; Morgan et al. 2006; Verdejo-Garcia et al. 2007; Zermatten et al. 2005), suggesting at least partially overlapping neurobiological mechanisms. Impulsivity is a heterogeneous behavioral construct that can be dissociated into two main subtypes, i.e. impulsive choice and impulsive action (Dalley et al. 2011; Eagle and Baunez 2010; Evenden 1999; Pattij and Vanderschuren 2008; Winstanley 2011). Neuropharmacological manipulations of monoaminergic activity have consistently reported a differential contribution of DA, NA and 5-HT in the modulation of these two dimensions of impulsivity (Baarendse and Vanderschuren 2012; Dalley et al. 2011; Pattij and Vanderschuren 2008; Van Gaalen et al. 2006a; -2006b). In the present study, we showed that impulsive action and decision making in the rGT are dissociable at the neuropharmacological level. Consistent with previous findings in the 5-CSRTT (Baarendse and Vanderschuren 2012; Cole and Robbins 1987; Paterson et al. 2011; Sun et al. 2012; Van Gaalen et al. 2006a), the amount of premature responses, i.e. responses made within the intertrial interval (ITI) before illumination of the stimulus lights, were significantly increased by amphetamine and GBR12909 in the rGT. These results indicate that impulsive action can be used as an independent additional measure of task performance in the rGT. Selective inhibition of NA reuptake by atomoxetine reduced premature responding. A decrease in impulsive action by atomoxetine has been shown before in the 5-CSRTT, especially under circumstances when task performance is suboptimal due to demanding task conditions or inherent high baseline levels of impulsive action (Baarendse and Vanderschuren 2012; Blondeau and Dellu-Hagedorn 2007; Fernando et al. 2012; Navarra et al. 2008; Paine et al. 2007; Paterson et al. 2011; Robinson et al. 2008). Comparable to its effects in the 5-CSRTT, atomoxetine increased errors of omission, suggesting that increased NA neurotransmission results in a more conservative, but somewhat slower task performance. Interestingly, combined DA and NA transporter blockade increased premature responding, pointing towards a primary role of DA in impulse control. The alterations in impulsive action induced by DA or NA transporter blockade was not accompanied by pronounced changes in choice behavior, suggesting that the neurobehavioral pathways underlying impulse control and decision making are at least partially independent.
In summary, the neuropharmacological manipulations presented here indicate a differential contribution of monoaminergic neurotransmission in the modulation of impulse control and decision making measured in the rGT. Inhibition of the reuptake of DA or NA altered impulsive action, whereas it did not influence choice for the advantageous versus disadvantageous options in the rGT. The current study, therefore, supports the clinical observations that impulsivity and decision making can manifest independently (Jollant et al. 2005; Mavaddat et al. 2000; Van der Plas et al. 2009). Combined inhibition of the reuptake of DA and NA impaired choice behavior in the rGT, suggesting important, but complementary or redundant roles of DA and NA neurotransmission in decision making processes based on probability of reward and punishment. This latter finding is of particular relevance to neuropsychiatric disorders that have been associated with impairments in decision making (Bark et al. 2005; Bechara and Damasio 2002; Brand et al. 2004; -2005; Cavedini et al. 2010; Ernst et al. 2003; Garon et al. 2006; Schutter et al. 2011). Improving our knowledge of the neural mechanisms involved in decision making may contribute to a better understanding of the psychiatric disorders in which this process is compromised.
Acknowledgements
This study was supported by the National Institute on Drug Abuse Grant R01 DA022628 (L.J.M.J.V.).
References
- Arnsten AFT. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Through the looking glass: differential noradrenergic modulation of prefrontal cortical function. Neural Plasticity. 2000;7:133–46. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Baarendse PJJ, Vanderschuren LJMJ. Dissociable effects of monoamine reuptake inhibitors on distinct forms of impulsive behavior in rats. Psychopharmacology. 2012;219:313–326. doi: 10.1007/s00213-011-2576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bark R, Dieckmann S, Bogerts B, Northoff G. Deficit in decision making in catatonic schizophrenia: an exploratory study. Psychiatry Res. 2005;134:131–141. doi: 10.1016/j.psychres.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (partI): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Blondeau C, Dellu-Hagedorn F. Dimensional analysis of ADHD subtypes in rats. Biol Psychiatry. 2007;61:1340–1350. doi: 10.1016/j.biopsych.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Boureau Y-L, Dayan P. Opponency revisited: competition and cooperation between dopamine and serotonin. Neuropsychopharmacology. 2010;36:74–97. doi: 10.1038/npp.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Brand M, Labudda K, Kalbe E, Hilker R, Emmans D, Fuchs G, Kessler J, Markowitsch HJ. Decision-making impairments in patients with Parkinson’s disease. Behav Neurol. 2004;15:77–85. doi: 10.1155/2004/578354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Kalbe E, Labudda K, Fujiwara E, Kessler J, Markowitsch HJ. Decision-making impairments in patients with pathological gambling. Psychiatry Res. 2005;133:91–99. doi: 10.1016/j.psychres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brand M, Recknor EC, Grabenhorst F, Bechara A. Decisions under ambiguity and decisions under risk: correlations with executive functions and comparisons of two different gambling tasks with implicit and explicit rules. J Clin Exp Neuropsychol. 2007;29:86–99. doi: 10.1080/13803390500507196. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Cavedini P, Riboldi G, Keller R, D’annucci A, Bellodi L. Frontal lobe dysfunction in pathological gambling patients. Biol. Psychiatry. 2002;51:334–341. doi: 10.1016/s0006-3223(01)01227-6. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology. 1987;91:458–466. doi: 10.1007/BF00216011. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Forebrain norepinephrine: role in controlled information processing in the rat. Neuropsychopharmacology. 1992;7:129–142. [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Cools R, Nakamura K, Daw ND. Serotonin and dopamine: unifying affective, activational, and decision functions. Neuropsychopharmacology. 2011;36:98–113. doi: 10.1038/npp.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O’Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J Neurosci. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–84. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dayan P, Huys QJ. Serotonin, inhibition, and negative mood. PLoS Comput Biol. 2008;4:e4. doi: 10.1371/journal.pcbi.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk F, Walton ME, Jennings KA, Sharp T, Rushworth MFS, Bannerman DM. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology. 2005;179:587–596. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev. 2010;34:50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Grant SJ, London ED, Contoreggi CS, Kimes AS, Spurgeon L. Decision making in adolescents with behavior disorders and adults with substanceabuse. Am J Psychiatry. 2003;160:33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fernando AB, Economidou D, Theobald DE, Zou MF, Newman AH, Spoelder M, Caprioli D, Moreno M, Hipólito L, Aspinall AT, Robbins TW, Dalley JW. Modulation of high impulsivity and attentional performance in rats by selective direct and indirect dopaminergic and noradrenergic receptor agonists. Psychopharmacology. 2012;219:341–52. doi: 10.1007/s00213-011-2408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology. 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008a;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Floresco SB, St. Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbicstriatal circuits subserving different forms of costbenefit decision making. Cogn Affect Behav Neurosci. 2008b;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Jentsch JD. Pharmacological enhancement of memory and executive functioning in laboratory animals. Neuropsychopharmacology. 2011;36:227–250. doi: 10.1038/npp.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH, Muris P. Individual differences in decision making. Personality and Individual Differences. 2005;39:991–998. [Google Scholar]
- Franken IH, van Strien JW, Nijs I, Muris P. Impulsivity is associated with behavioral decision-making deficits. Psychiatry Res. 2008;158:155–163. doi: 10.1016/j.psychres.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Gallagher DA, O’Sullivan SS, Evans AH, Lees AJ, Schrag A. Pathological gambling in Parkinson’s disease: risk factors and differences from dopamine dysregulation. An analysis of published case series. Mov Disord. 2007;22:1757–1763. doi: 10.1002/mds.21611. [DOI] [PubMed] [Google Scholar]
- Garon N, Moore C, Waschbusch DA. Decision making in children with ADHD only, ADHD-anxious/depressed, and control children using a child version of the Iowa gambling task. J Atten Disord. 2006;9:607–619. doi: 10.1177/1087054705284501. [DOI] [PubMed] [Google Scholar]
- Grant JE, Potenza MN. Treatments for pathological gambling and other impulse control disorders. In: Nathan P, Gorman J, editors. A Guide to Treatments That Work. Oxford University Press; Oxford: 2007. pp. 561–579. [Google Scholar]
- Hanson KL, Luciana M, Sullwold K. Reward-related decision-making deficits and elevated impulsivity among MDMA and other drug users. Drug Alcohol Depend. 2008;96:99–110. doi: 10.1016/j.drugalcdep.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Rosen J. Impulsivity. J Psychopharmacol. 2000;14:S39–44. doi: 10.1177/02698811000142S106. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Yechiam E, Murphy RR, Queller S, Stout JC. Motivational processes and autonomic responsivity in Asperger’s disorder: evidence from the Iowa Gambling Task. J Int Neuropsychol Soc. 2006;12:668–676. doi: 10.1017/S1355617706060802. [DOI] [PubMed] [Google Scholar]
- Jollant F, Bellivier F, Leboyer M, Astruc B, Torres S, Verdier R, Castelnau D, Malafosse A, Courtet P. Impaired decision making in suicide attempters. American Journal of Psychiatry. 2005;162:304–310. doi: 10.1176/appi.ajp.162.2.304. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience. 2010;166:1023–1035. doi: 10.1016/j.neuroscience.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Wooderson S, Mataix-Cols D, David R, Speckens A, Phillips ML. Decision making and set shifting impairments are associated with distinct symptom dimensions in obsessive-compulsive disorder. Neuropsychology. 2006;20:409–419. doi: 10.1037/0894-4105.20.4.409. [DOI] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Knol DL, Sergeant JA. Decision-making in ADHD: sensitive to frequency but blind to the magnitude of penalty? J. Child Psychol. Psychiatry. 2008;49:712–722. doi: 10.1111/j.1469-7610.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- Malloy-Diniz L, Fuentes D, Leite WB, Correa H, Bechara A. Impulsive behavior in adults with attention deficit/hyperactivity disorder: characterization of attentional, motor and cognitive impulsiveness. J Int Neuropsychol Soc. 2007;13:693–698. doi: 10.1017/S1355617707070889. [DOI] [PubMed] [Google Scholar]
- Mavaddat N, Kirkpatrick PJ, Rogers RD, Sahakian BJ. Deficits in decision-making in patients with aneurysms of the anterior communicating artery. Brain. 2000;123:2109–2117. doi: 10.1093/brain/123.10.2109. [DOI] [PubMed] [Google Scholar]
- McDonald J. Handbook of biological statistics. Vol. 2. Sparky House Publishing; Baltimore, Ed: 2009. [Google Scholar]
- Mitchell MR, Vokes CM, Blankenship AL, Simon NW, Setlow B. Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology. 2011;218:703–712. doi: 10.1007/s00213-011-2363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ, Impallomeni LC, Pirona A, Rogers RD. Elevated impulsivity and impaired decision-making in abstinent ecstasy (MDMA) users compared to polydrug and drug-naïve controls. Neuropsychopharmacology. 2006;31:1562–1573. doi: 10.1038/sj.npp.1300953. [DOI] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- O’Carroll RE, Papps BP. Decision making in humans: the effect of manipulating the central noradrenergic system. J Neurol Neurosurg Psychiatry. 2003;74:376–378. doi: 10.1136/jnnp.74.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell JM, Marek GJ, Seiden LS. Antidepressant effects assessed using behavior maintained under a differential reinforcement- of-low-rate (DRL) operant schedule. Neurosci Biobehav Rev. 2005;29:785–798. doi: 10.1016/j.neubiorev.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Young MB, Lestini MM, Schutsky K, Thomas SA. Redundant catecholamine signaling consolidates fear memory via phospholipase C. J Neurosci. 2012;32:1932–1941. doi: 10.1523/JNEUROSCI.5231-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA. Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biol Psychiatry. 2007;62:687–93. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Ricciardi J, Wetzler C, Hanania T. Sub-optimal performance in the 5-choice serial reaction time task in rats was sensitive to methylphenidate, atomoxetine and D-amphetamine, but unaffected by the COMT inhibitor tolcapone. Neurosci Res. 2011;69:41–50. doi: 10.1016/j.neures.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Raylu N, Oei TP. Pathological gambling. A comprehensive review. Clin Psychol Rev. 2002;22:1009–1061. doi: 10.1016/s0272-7358(02)00101-0. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: commentary on Berridge (2006) Psychopharmacology. 2007;191:433–437. doi: 10.1007/s00213-006-0528-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Rogers RD. The roles of dopamine and serotonin in decision making: evidence from pharmacological experiments in humans. Neuropsychopharmacology. 2011;36:114–32. doi: 10.1038/npp.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Bokhoven I, Vanderschuren LJ, Lochman JE, Matthys W. Risky decision making in substance dependent adolescents with a disruptive behavior disorder. J Abnorm Child Psychol. 2011;39:333–339. doi: 10.1007/s10802-010-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seedat S, Kesler S, Niehaus DJ, Stein DJ. Pathological gambling behaviour: emergence secondary to treatment of Parkinson’s disease with dopaminergic agents. Depress Anxiety. 2000;11:185–186. doi: 10.1002/1520-6394(2000)11:4<185::AID-DA8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Sevy S, Hassoun Y, Bechara A, Yechiam E, Napolitano B, Burdick K, Delman H, Malhotra A. Emotion-based decision-making in healthy subjects: short-term effects of reducing dopamine levels. Psychopharmacology. 2006;188:228–235. doi: 10.1007/s00213-006-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurman B, Horan WP, Nuechterlein KH. Schizophrenia patients demonstrate a distinctive pattern of decision-making impairment on the Iowa Gambling Task. Schizophr Res. 2005;72:215–224. doi: 10.1016/j.schres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology. 2009;34:2208–2217. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA, Bañuelos C, Vokes CM, Taylor AB, Haberman RP, Bizon JL, Setlow B. Dopaminergic modulation of risky decision-making. J Neurosci. 2011;31:17460–17470. doi: 10.1523/JNEUROSCI.3772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirviö J, Jäkälä P, Mazurkiewicz M, Haapalinna A, Riekkinen P, Jr, Riekkinen PJ. Dose- and parameter-dependent effects of atipamezole, an alpha 2-antagonist, on the performance of rats in a five-choice serial reaction time task. Pharmacol Biochem Behav. 1993;45:123–129. doi: 10.1016/0091-3057(93)90095-b. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic regulation of risk based decision-making. Neuropsychopharmacology. 2009;34:681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- Sun H, Cocker PJ, Zeeb FD, Winstanley CA. Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology. 2012;219:285–301. doi: 10.1007/s00213-011-2419-9. [DOI] [PubMed] [Google Scholar]
- Tops M, Russo S, Boksem MA, Tucker DM. Serotonin: modulator of a drive to withdraw. Brain Cogn. 2009;71:427–436. doi: 10.1016/j.bandc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- Van den Brink W. Evidence-based pharmacological treatment of substance use disorders and pathological gambling. Curr Drug Abuse Rev. 2012;5:3–31. doi: 10.2174/1874473711205010003. [DOI] [PubMed] [Google Scholar]
- Van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol. 2009;31:706–719. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaalen MM, Brueggeman RJ, Bronius PFC, Schoffelmeer ANM, Vanderschuren LJMJ. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology. 2006a;187:73–85. doi: 10.1007/s00213-006-0396-1. [DOI] [PubMed] [Google Scholar]
- Van Gaalen MM, van Koten R, Schoffelmeer ANM, Vanderschuren LJMJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006b;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Rivas-Pérez C, Vilar-López R, Pérez-García M. Strategic self-regulation, decision-making and emotion processing in poly-substance abusers in their first year of abstinence. Drug Alcohol Depend. 2007;86:139–46. doi: 10.1016/j.drugalcdep.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63:969–973. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol. 2011;164:1301–1321. doi: 10.1111/j.1476-5381.2011.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–2343. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Winstanley CA. Lesions of the basolateral amygdala and orbitofrontal cortex differentially affect acquisition and performance of a rodent gambling task. J Neurosci. 2011;31:2197–2204. doi: 10.1523/JNEUROSCI.5597-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zermatten AM, Van der Linden MP, d’Acremont MM, Jermann FM, Bechara AP. Impulsivity and decision making. Journal of Nervous and Mental Disease. 2005;193:647–650. doi: 10.1097/01.nmd.0000180777.41295.65. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Wang GB, Zhao LY, Sun LL, Wang J, Wu P, Lu L, Shi J. Clonidine improved laboratory-measured decision-making performance in abstinent heroin addicts. PLos One. 2012;7:e29084. doi: 10.1371/journal.pone.0029084. [DOI] [PMC free article] [PubMed] [Google Scholar]