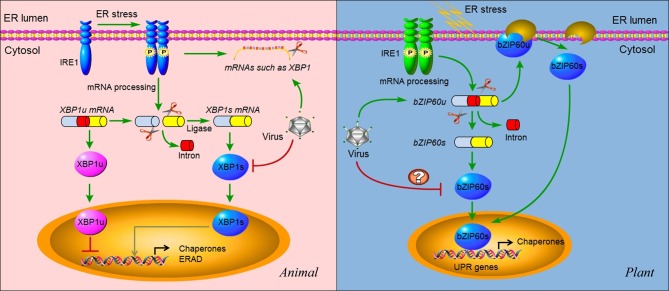
Figure 4.
IRE1 signaling and virus infection in animals and plants. In animals, IRE1 oligomerizes in the plane of the ER membrane in stressed cells, leading to trans-autophosphorylation and activation. Activated IRE1 mediates the sequence-specific cleavage of the XBP1 mRNA in higher eukaryotes, deleting a small RNA fragment (intron) and finally producing a spliced mRNA (XBP1s) with a frame shift in the coding sequence. Spliced XBP1s encodes a potent transcriptional activator (XBP1s), whereas the unspliced XBP1 mRNA (XBP1u) encodes an inhibitor of the UPR (XBP1u). In mammals, it seems that XBP1s regulates a subset of UPR genes that promote ERAD of misfolded proteins and refold proteins. In cultured Drosophila melanogaster cells, activated IRE1 can promote the cleavage of mRNAs, including XBP1 mRNA, leading to their degradation. This reduces the load on the stressed ER and might facilitate reprogramming of the ER-associated protein synthesis and translocation machinery. In cells infected by viruses such as HCV, the IRE1 pathway is manipulated by the virus via repressing the transcriptional activity of XBP1s. In addition, some viruses might also promote the IRE1-dependent mRNA decay as a means to manipulate the IRE1 pathway. In plants, IRE1 homologs were detected in the genomes of Arabidopsis and rice a decade ago. However, the target of IRE1 was not identified until 2011. The mRNA of transcriptional factor bZIP60 is the substrate of IRE1 in plants. Similar to XBP1 in animals, unspliced bZIP60 (bZIP60u) is processed by activated IRE1. The protein product (bZIP60s) translated from the spliced bZIP60 (bZIPs) is translocated into the nucleus to activate the expression of UPR genes such as chaperones. Different from XBP1u, plant bZIP60u protein, translated from bZIP60u mRNA, is retained in the ER membrane. Sensing unfolded proteins in the ER lumen, bZIP60u undergoes a proteolytic processing, releasing bZIP60s. A recent study has shown that the expression of bZIP60 was increased by PVX infection. However, the roles of the UPR pathway in virus infection have only begun to be investigated in plants. Critical unanswered questions need to be addressed in the future, such as whether viruses modulate the IRE1 pathway via inhibiting the transcriptional activity of bZIP60s (indicated by “?”).