Background: Previously we demonstrated that metformin stimulates GLUT4 through AMPK in skeletal muscle system. However, it was not clear how GLUT4 translocation is affected by metformin in adipocyte system.
Results: Metformin stimulates AMPK to phosphorylate Cbl and induce CAP expression, thus modulating GLUT4 translocation.
Conclusion: Cbl and CAP are involved in metformin-induced AMPK-mediated GLUT4 translocation.
Significance: Cbl and CAP are downstream effectors of metformin on GLUT4 translocation.
Keywords: AMP-activated Kinase (AMPK), Diabetes, Glucose, GLUT4, Metabolism, Metformin
Abstract
Metformin is a leading oral anti-diabetes mellitus medication and is known to stimulate GLUT4 translocation. However, the mechanism by which metformin acts is still largely unknown. Here, we showed that short time treatment with metformin rapidly increased phosphorylation of Cbl in an AMP-activated protein kinase (AMPK)-dependent fashion in 3T3-L1 preadipocytes. Metformin also increased phosphorylation of Src in an AMPK-dependent manner. Src inhibition blocked metformin-mediated Cbl phosphorylation, suggesting that metformin stimulates AMPK-Src-Cbl axis pathway. In addition, long term treatment with metformin stimulated the expression of Cbl-associated protein (CAP) mRNA and protein. Long term treatment with metformin stimulated phosphorylation of c-Jun N-terminal kinase (JNK) and its downstream molecule c-Jun, which is a critical molecule for CAP transcription. Knockdown of AMPK and JNK blocked metformin-induced expression of CAP, implying that metformin stimulates AMPK-JNK-CAP axis pathway. Moreover, AMPK knockdown attenuated metformin-induced Cbl/CAP multicomplex formation, which is critical for GLUT4 translocation. A colorimetric absorbance assay demonstrated that metformin-induced translocation of GLUT4 was suppressed in CAP or Cbl knockdown cells. Furthermore, the promoter activity of CAP was increased by metformin in an AMPK/JNK-dependent fashion. In summary, these results demonstrate that metformin modulates GLUT4 translocation by regulating Cbl and CAP signals via AMPK.
Introduction
Metformin is an oral anti-diabetes mellitus medication of the biguanide class. Metformin originates from the French lilac (Galega officinalis), a plant known to reduce the symptoms of diabetes mellitus (1). Metformin has many effects on insulin sensitivity in muscle and liver, including a decrease in hepatic glucose production, an increase in peripheral glucose utilization, and positive effects on insulin receptor expression and tyrosine kinase activity (2–4). Metformin also ameliorates insulin resistance by inducing glucose transporter 4 (GLUT4)2 translocation (5–7). However, it is still unclear how metformin achieves GLUT4 translocation.
AMP-activated protein kinase (AMPK) is an enzyme that plays a role in cellular energy homeostasis. AMPK is activated when cellular energy is depleted (8). Upon phosphorylation at Thr172 of the catalytic subunit, AMPK accelerates ATP-generating catabolic pathways, including glycolysis and fatty acid oxidation (9–11), while simultaneously reducing ATP-consuming anabolic pathways (cholesterol, fatty acid, and triacylglycerol synthesis) (12). In addition to its roles in energy homeostasis, activation of AMPK through physiological stimulation, such as muscle contraction or by the pharmacologic activator, 5-aminoimidazole-4-carboxy-amide-1-d-ribofuranoside (AICAR), leads to a significant increase in glucose uptake, which is mediated by translocation of GLUT4 (13, 14). GLUT4 is highly expressed in adipose tissue and skeletal muscle. GLUT4 is a major mediator of glucose removal from the circulation and functions as a key regulator of whole body glucose homeostasis (15). However, the precise mechanism by which metformin achieves its hypoglycemic effect has not yet been elucidated.
The stimulation of glucose uptake requires translocation of GLUT4 protein from intracellular storage sites to the cell surface. Insulin-induced translocation of GLUT4 requires the activity of phosphatidylinositol (PI) 3-kinase (16). PKB/Akt mediates the stimulation of glucose transport by insulin in rat adipocytes (17) and L6 muscle cells (18). PI3-kinase lipid products binds to the pleckstrin homology domain of the PKB/Akt and stimulates its activity (19). Despite the evidence supporting an important role for the PI3-kinase pathway, activation of this enzyme is not enough for glucose transport. The Cbl-CAP-CrkII-C3G-TC10 pathway is another route for GLUT4 translocation. c-Cbl is the cellular homolog of the transforming v-Cbl oncogene (20). Cbl contains numerous tyrosine residues, which could serve as docking sites for multiple Src homology (SH)2-containing signaling molecules upon phosphorylation. Cbl consists of an N-terminal variant SH2 domain, a RING finger domain, multiple proline-rich stretches, several potential tyrosine phosphorylation sites, and a conserved ubiquitin-associated domain (21). CAP is a versatile adaptor protein that contains three C-terminal SH3 binding domains and one sorbin-like region. The functional association of CAP with the proto-oncogene product c-Cbl is mediated by the C-terminal SH3 binding domain (22–25). CAP constitutively interacts with Cbl via its C-terminal SH3 domain. Upon Cbl phosphorylation, the Cbl/CAP complex migrates to caveolin-enriched lipid rafts, as a result of the interaction of the SoHo domain on CAP with the lipid raft-associated protein, flotillin-1 (26). This leads to recruitment of the CrkII/C3G complex to the microdomain of the plasma membrane, where C3G, a guanyl nucleotide exchange factor, activates the small GTP-binding protein TC10. Activation of TC10 has been shown to occur independently of the PI3-kinase pathway. More importantly, it is crucial to insulin-stimulated GLUT4 translocation (27).
In this study, we found that metformin rapidly phosphorylated Cbl, chronically induced CAP expression via AMPK, and modulated GLUT4 translocation by regulating Cbl/CAP-associated multicomplex formation.
EXPERIMENTAL PROCEDURES
Reagents
Anti-phospho-JNK (Thr183/Tyr185), anti-JNK, anti-phospho-c-Jun (Ser63), and anti-c-Jun antibodies were purchased from Cell Signaling Technology. Anti-AMPKα2, anti-phospho-AMPK (Thr172), anti-Src, anti-CAP, and anti-Cbl antibodies were purchased from Millipore-Upstate. Anti-phospho-Cbl antibodies (Tyr731) were purchased from Abcam. Anti-phospho-Src antibodies (Tyr307) were purchased from Epitomics Inc. (Burlingame, CA). Anti-CRKII and anti-flotillin-1 antibodies were purchased from Santa Cruz Biotechnology. Anti-actin antibody and PP2 (src inhibitor, 4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine were purchsed from Sigma. Horseradish peroxidase-conjugated secondary antibodies were obtained from Assay Designs and Stressgen (Ann Arbor, MI). Metformin, compound C, and AICAR were obtained from Calbiochem.
Cell Culture
3T3-L1 prepreadipocytes were cultured in DMEM containing 25 mm glucose, 10% fetal serum at 37 °C, and 5% CO2. L6 muscle cells expressing c-myc epitope tagged GLUT4 (L6myc cells) were maintained in myoblast monolayer culture in α-MEM containing 10% (v/v) FBS and 1% (v/v) antibiotic-antimycotic solution (100 units/ml penicillin G, 10 μg/ml streptomycin, and 25 mg/ml amphotericin B) in an atmosphere of 5% CO2 at 37 °C. For differentiation into myotubes, L6 myoblasts were plated in medium containing 2% (v/v) FBS at a density of 104 cells/ml to allow spontaneous myoblast fusion. The medium was changed every 48 h, and myotubes were ready for experimentation at 6–8 days after plating. Prior to experiments, cells were incubated with serum-free α-MEM supplemented with 25 mm glucose for 2 h.
Immunoblot Analysis
Cells were grown on 6-well plates and serum-starved for 36 h prior to treatment with the indicated agents. Following treatment of the cells, the medium was aspirated, and the cells were washed twice in ice-cold PBS and lysed in 100 μl of lysis buffer. Samples were then briefly sonicated, heated for 5 min at 95 °C, and centrifuged for 5 min. Supernatants were electrophoresed on SDS-polyacrylamide (8%) gels and transferred to polyvinylidene difluoride membranes. Blots were incubated overnight at 4 °C with primary antibodies and then washed six times in Tris-buffered saline/0.1% Tween 20 prior to a 1-h incubation with horseradish peroxidase-conjugated secondary antibodies at room temperature. Blots were then visualized via ECL (Amersham Biosciences). In some cases, the blots were stripped and reprobed using other antibodies.
Immunoprecipitation
The amount of proteins was determined using the Bradford method. Cellular protein (1000 μg) was mixed with 1 μg of anti-Cbl antibody and incubated overnight at 4 °C. Then, 10 μl of protein A-Sepharose (Amersham Biosciences) was added to the samples and incubated for another 3 h at 4 °C. After incubation, the samples were washed three times with washing buffer (25 mm HEPES, 5 mm EDTA, 1% Triton X-100, 50 mm NaF, 150 mm NaCl, 10 mm phenylmethylsulfonyl fluoride, 1 μm leupeptin, 1 μm pepstatin, and 1 μm aprotinin A (pH 7.2)). The washed samples were resuspended in SDS sample buffer (125 mm Tris-HCl (pH 6.8), 20% (v/v) glycerol, 4% (w/v) SDS, 100 mm dithiothreitol, and 0.1% (w/v) bromphenol blue) and heated at 100 °C for 5 min prior to electrophoresis.
Silencing AMPKα2, JNK, Cbl, and CAP
3T3-L1 preadipocytes were seeded in 6-well plates and allowed to grow to a confluence of 70% over 24 h. Transient transfections were performed with transfection reagent (Lipofectamine 2000; Invitrogen) according to the manufacturer's protocol. Briefly, AMPKα2 (NM_001013367; Dharmacon), JNK (NM_016700; Dharmacon), Cbl (XM576396; Genolution Pharmaceuticals Inc., Seoul, Korea), CAP (U_58883; Genolution Pharmaceuticals), and nontargeted control siRNAs were designed. Five μl of siRNA and 5 μl of transfection reagent (Lipofectamine 2000) were each diluted first with 95 μl of reduced serum medium (Opti-MEM; Invitrogen) and then mixed. The mixtures were allowed to incubate for 30 min at room temperature and were then added dropwise to each culture well, which contained 800 μl of reduced serum medium (Opti-MEM, final siRNA concentration (100 nm); Invitrogen). Four hours after transfection, the medium was changed with fresh complete medium. Cells were cultivated for 24 h and lysed, and the expression of AMPKα2 protein was measured by Western blotting.
RT-PCR
First strand cDNA synthesis was performed using 1 μg of total RNA isolated from 3T3-L1 preadipocytes at 55 °C for 20 min using the Thermoscript II one-step RT-PCR Kit (Invitrogen). Amplification of cDNA was carried out in the same tube using the Gene Amp System 9700 thermocycler (Applied Biosystems). Heating to 94 °C for 5 min was used to inactivate the reverse transcriptase. The following PCR conditions were used: 27 cycles for 30 s at 94 °C, 30 s at 56 °C, and 30 s at 72 °C, followed by 7 min at 72 °C. The number of PCR cycles used was optimized to ensure amplification at the exponential phase. Ten-μl samples from each RT-PCR product were removed and analyzed by agarose gel electrophoresis. Bands were stained with ethidium bromide and visualized under ultraviolet light. Band intensity quantification was determined using a gel documentation system (Gene Genius, Syngene, UK). The following primers were used: CAP-sense (5′-TACATCGAAGGGGAGAAAGTG G-3′) and CAP-antisense (5′-TCTTTATCATCGTGCCGTCTC C-3′); and actin-sense (5′-ATTTGGTCGTATTGGGCGCCTGGTCACC-3′) and actin antisense (5′-GAAGATGGTGATGGGATTTC-3′).
Immunodetection of GLUT4myc
Cell surface GLUT4myc was quantified using antibody-coupled colorimetric absorbance assays, as described previously (28). Briefly, following stimulation with insulin or metformin, L6-GLUT4myc-tagged myoblasts were exposed to polyclonal anti-myc antibody (1:100) for 60 min, fixed with 4% paraformaldehyde for 10 min, and incubated with peroxidase-conjugated goat anti-rabbit IgG (1:1000) for 1 h. Cells were then washed six times, and 1 ml of o-phenylenediamine dihydrochloride (0.4 mg/ml) was added to the cells and incubated for 30 min. The absorbance of the supernatant was measured at 492 nm.
Plasmid Construction of CAP Promoter
A 378-bp genomic fragment (from −513 to −135) containing the CAP promoter was cloned into an empty pGL3-basic vector (pGL3; Promega,) between the Kpn1 and Sac1 sites (Promega). Genomic DNA from 3T3-L1 preadipocyte subjects was amplified by PCR (forward primer, 5′-GGGTACCAACCACAGCATAGTTAGG-3′; reverse primer 5′-GAGCTCGCAAACAGAGCTGAACTAG-3′). Kpn1- and Sac1-digested products were ligated into a linearized pGL3-basic vector containing the P1 promoter (pGL3-CAP). A positive control construct was made by cloning a pCMV5 promoter into the pGL3-basic vector (pGL3-pCMV). All constructs were verified by direct sequencing.
Luciferase Assay
After transfection, cells were harvested, and the extracts were prepared using reporter lysis buffer (Promega). Luciferase activity in the cell lysates was measured using a dual luciferase assay kit (Promega) and an illuminometer (VICTORTM3; PerkinElmer Life Sciences). Each extract was assayed three times.
Data Analysis
Data are expressed as the means ± S.E. Image Gauge (version 3.12; Fujifilm, Tokyo, Japan) was used for analysis of band intensity. One-way ANOVA was used followed by a Holm-Sidak multiple range test for comparison between groups. p values < 0.05 were considered statistically significant.
RESULTS
Short Time Treatment with Metformin Induces Phosphorylation of Cbl in an AMPK-dependent Manner
To understand the role of AMPK in GLUT4 translocation, we evaluated the effect of AICAR, an AMPK activator, on phosphorylation of Cbl in 3T3-L1 preadipocytes. Administration of AICAR resulted in a time-dependent increase in the level of phosphorylation of Cbl (Fig. 1A). To determine the physiologic relevance in regard to Cbl phosphorylation, we assessed the effects of metformin on Cbl phosphorylation. Administration of metformin induced a dose-dependent increase in the level of phosphorylation of Cbl in 3T3-L1 preadipocytes (Fig. 1B). One mm metformin is enough for Cbl phosphorylation. The level of phosphorylation of Cbl was significantly suppressed by treatment with compound C, an AMPK inhibitor (Fig. 1C). Cbl phosphorylation was increased by the treatment with 500 μm metformin time-dependently (Fig. 1D). Taken together, these results demonstrate that metformin regulates phosphorylation of Cbl in an AMPK-dependent fashion in 3T3-L1 preadipocytes.
FIGURE 1.
AICAR and metformin induce phosphorylation of c-Cbl in an AMPK-dependent manner. A, 3T3-L1 preadipocytes were stimulated for the indicated times with 1 mm AICAR. Cell lysates (25 μg) were analyzed via Western blotting (IB) using an anti-phospho-Cbl (Tyr731) antibody. Blotting with anti-Cbl antibody was used as a protein-loading control. B, 3T3-L1 preadipocytes were stimulated for the indicated doses of metformin for 1 h. Cell lysates (25 μg) were analyzed via Western blotting using an anti-phospho-Cbl (Tyr731) antibody. Blotting with anti-Cbl antibody was used as a protein-loading control. C, 3T3-L1 preadipocytes were stimulated for 30 min with 1 mm metformin in the presence of compound C (2 μm). Cell lysates (25 μg) were analyzed via Western blotting with an anti-phospho-Cbl (Tyr731) antibody. Blotting with the anti-Cbl antibody was used as a protein-loading control. D, 3T3-L1 preadipocytes were stimulated with 500 μm metformin for the indicated times. Cell lysates (25 μg) were analyzed via Western blotting with an anti-phospho-Cbl (Tyr731) antibody. Blotting with the anti-Cbl antibody was used as a protein-loading control. Data in the bar graphs (A–D) represent the mean ± S.E. (error bars) values of the ratios of densities (p-Cbl/Cbl) for at least three individual Western blotting experiments. *, p < 0.05 versus basal values.
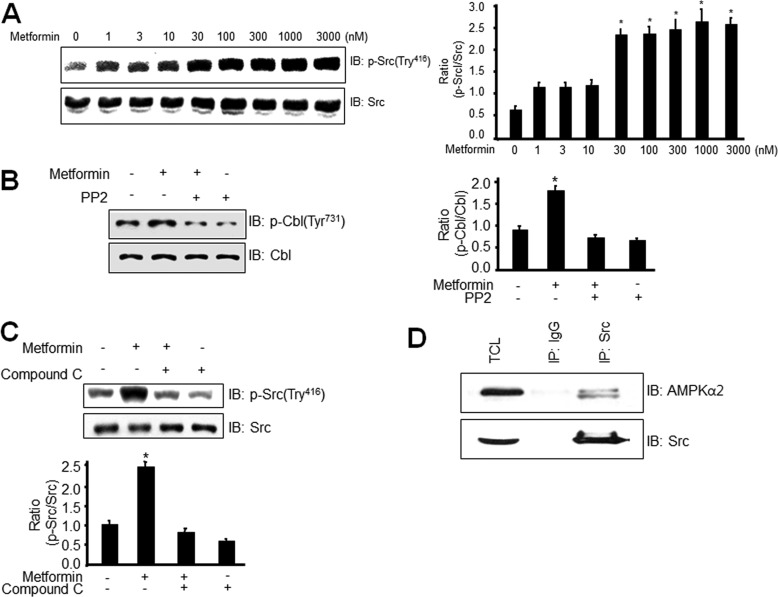
Metformin Stimulates Phosphorylation of Src in an AMPK-dependent Manner
To further characterize the role of AMPK on metformin-induced Cbl phosphorylation, we examined the effects of metformin on phosphorylation of Src, a nonreceptor tysosine kinase. In this experiment, we investigated various doses of metformin on the phosphorylation of Src. The nanomolar ranges of metformin increased the phosphorylation of Src. Considering the fact that pharmacological doses of metformin are in the nanomolar ranges, this result suggests that the metformin effect is physiologically relevant (Fig. 2A). To examine the involvement of Src in Cbl phosphorylation, we investigated the effect of Src inhibitor PP2 on Cbl phosphorylation. PP2 blocked metformin-induced Cbl phosphorylation (Fig. 2B), indicating that Src may be involved in metformin-mediated Cbl phosphorylation. To verify the role of AMPK, we assessed the effects of AMPK inhibitor, compound C, on Src phosphorylation. Inhibition of AMPK suppressed phosphorylation of Src, suggesting that AMPK may be involved in metformin-mediated Src phosphorylation (Fig. 2C). To explain the mechanism of AMPK-mediated Cbl phosphorylation, we examined the interaction between AMPKα2 and Src. We showed that Src interacted with AMPKα2 in 3T3L1 cells (Fig. 2D). These results demonstrate that metformin stimulates Cbl phosphorylation by regulating AMPK-mediated Src activity.
FIGURE 2.
Metformin stimulates phosphorylation of Src in an AMPK-dependent manner. A, 3T3-L1 preadipocytes were stimulated for the indicated doses with metformin for 6 h. Cell lysates (25 μg) were analyzed via Western blotting (IB) with anti-phospho-Src antibody. Blotting with anti-Src antibody was used as a protein-loading control. Data in the bar graphs represent the mean ± S.E. (error bars) values of the ratios of densities (p-Src/Src) for at least three individual Western blotting experiments. *, p < 0.05 versus basal values. B, 3T3-L1 preadipocytes were stimulated for 30 min with 1 mm metformin in the presence of Src inhibitor PP2 (1 μm). Cell lysates (25 μg) were analyzed via Western blotting with an anti-phospho-Cbl antibody. Blotting with anti-Cbl antibodies was used as a protein-loading control. Data in the bar graphs represent the mean ± S.E. values of the ratios of densities (p-Cbl/Cbl) for at least three individual Western blot experiments. *, p < 0.05 versus basal values. C, 3T3-L1 preadipocytes were stimulated for 30 min with 1 mm metformin in the presence of compound C. Cell lysates (25 μg) were analyzed via Western blotting using an anti-phospho-Src antibody. Blotting with anti-Src antibody was used as a protein-loading control. Data in the bar graphs represent the mean ± S.E. values of the ratios of densities (p-Src/Src) for at least three individual Western blotting experiments. *, p < 0.05 versus basal values. D, 3T3-L1 preadipocytes were immunoprecipitated with the anti-Src antibody, followed by Western blotting using the anti-AMPKα2 or Src antibody. TCL, total cell lysates.
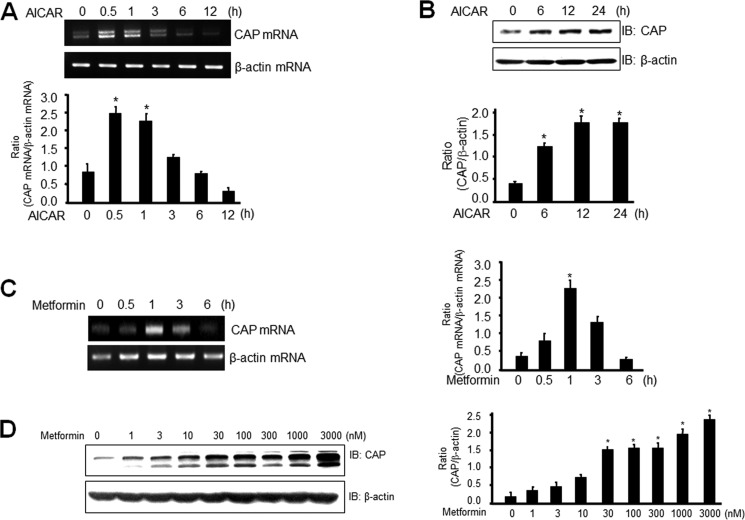
Metformin Induces CAP mRNA and Protein Levels in 3T3-L1 Preadipocytes
CAP is a multifunctional adaptor protein with three adjacent SH3 domains in the C terminus, which binds directly to the proline-rich domain of Cbl. Activation of CAP appears to be necessary for GLUT4 translocation (29). To better understand the role of AMPK in CAP expression, we evaluated the effect of AICAR on the expression of CAP. Administration of AICAR induced a time-dependent increase in the level of expression of CAP mRNA (Fig. 3A) and protein (Fig. 3B) in 3T3-L1 preadipocytes. To determine the physiologic relevance of CAP expression, we assessed the effects of metformin on expression of CAP. mRNA and protein levels of CAP were increased by treatment of 3T3-L1 preadipocytes with metformin (Fig. 3, C and D). Taken together, these results suggest that metformin regulates expression of CAP through AMPK in 3T3-L1 preadipocytes.
FIGURE 3.
Metformin induces CAP mRNA and protein levels in 3T3-L1 preadipocytes. A and C, total RNA was prepared for cells after 1 mm AICAR or 1 mm metformin treatment, and RT-PCR was conducted using specific primers. The PCR product was then gel run in 1% agarose and visualized in UV. β-Actin mRNA was employed as a positive control. Data in the bar graphs represent the mean ± S.E. (error bars) values of the ratios of densities (CAP mRNA/β-actin mRNA) for at least three individual Western blotting experiments. *, p < 0.05 versus basal values. B and D, 3T3-L1 preadipocytes were stimulated for the indicated times with 1 mm AICAR or the indicated doses of metformin for 6 h. Cell lysates (25 μg) were analyzed via Western blotting (IB) with an anti-CAP antibody. Blotting with the anti-β-actin antibody was used as a protein-loading control. The results shown are representative of three independent experiments. Data in the bar graphs represent the mean ± S.E. values of the ratios of densities (CAP/β-actin) for at least three individual Western blotting experiments. *, p < 0.05 versus basal values.
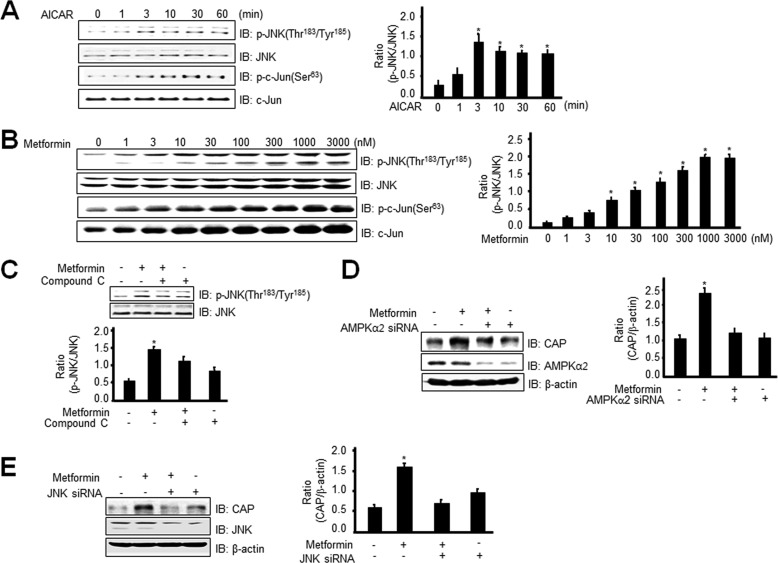
Metformin Stimulates CAP Expression through Phosphorylation of JNK in an AMPK-dependent Manner
To characterize further the signal pathway for metformin-mediated CAP expression, we examined the sequence of the CAP promoter. The promoter of CAP contains c-Fos and c-Jun binding sites (27). We examined the effects of metformin on phosphorylation of JNK. We observed that AICAR initiated an increase in JNK phosphorylation in 3T3-L1 preadipocytes (Fig. 4A). The phosphorylation of c-Jun, directly downstream of JNK, was also increased by AICAR treatment. Metformin increased the phosphorylation of JNK in a dose-dependent manner (Fig. 4B). To examine the roles of AMPK in the metformin-mediated signaling pathway, we assessed the effects of AMPK inhibition on JNK phosphorylation. Compound C blocked metformin-induced JNK phosphorylation (Fig. 4C). Moreover, metformin-induced CAP expression was blocked under the AMPKα2 knockdown condition (Fig. 4D). Knockdown of JNK blocked metformin-mediated CAP induction (Fig. 4E), suggesting that JNK is involved in CAP expression. These results demonstrate that metformin stimulates CAP expression through AMPK-mediated JNK phosphorylation.
FIGURE 4.
Metformin stimulates CAP expression through phosphorylation of JNK in an AMPK-dependent manner. A, 3T3-L1 preadipocytes were stimulated for the indicated times with 1 mm AICAR. Cell lysates (25 μg) were analyzed via Western blotting (IB) with an anti-phospho-JNK (Thr183/Tyr185) or anti-phospho c-Jun (Ser63) antibody. Blotting with the anti-JNK or c-Jun antibody was used as a protein-loading control. Data in the bar graphs represent the mean ± S.E. (error bars) values of the ratios of densities (p-JNK/JNK) for at least three individual Western blotting experiments. *, p < 0.05 versus basal values. B, 3T3-L1 preadipocytes were stimulated with the indicated doses of metformin for 6 h. Cell lysates (25 μg) were analyzed via Western blotting with an anti-phospho-JNK (Thr183/Tyr185) antibody. Blotting with the anti-JNK antibody was used as a protein-loading control. Data in the bar graphs represent the mean ± S.E. values of the ratios of densities (p-JNK/JNK) for at least three individual Western blotting experiments. *, p < 0.05 versus basal values. C, 3T3-Ll preadipocytes were stimulated for 30 min with 1 mm metformin in the presence of compound C. Cell lysates (25 μg) were analyzed via Western blotting with an anti-phospho-JNK (Thr183/Tyr185) antibody. Blotting with the anti-JNK antibody was used as a protein-loading control. Data in the bar graphs represent the mean ± S.E. values of the ratios of densities (p-JNK/JNK) for at least three individual Western blotting experiments. *, p < 0.05 versus basal values. D, 3T3-L1 preadipocytes were transiently transfected with AMPKα2 siRNA for 48 h, prior to 1 mm metformin treatment for 6 h. Cell lysates (25 μg) were analyzed via Western blotting using the anti-CAP or AMPKα2 antibody. Blotting with the anti-β-actin antibody was used as a protein-loading control. Data in the bar graphs represent the mean ± S.E. values of the ratios of densities (CAP/β-actin) for at least three individual Western blotting experiments. *, p < 0.05 versus basal values. E, 3T3-L1 preadipocytes were transiently transfected with JNK siRNA for 48 h, prior to 1 mm metformin treatment for 6 h. Cell lysates (25 μg) were analyzed via Western blotting with the anti-CAP or JNK antibody. Blotting with anti-β-actin antibody was used as a protein-loading control. Data in the bar graphs represent the mean ± S.E. values of the ratios of densities (CAP/β-actin) for at least three individual Western blot experiments. *, p < 0.05 versus basal values.
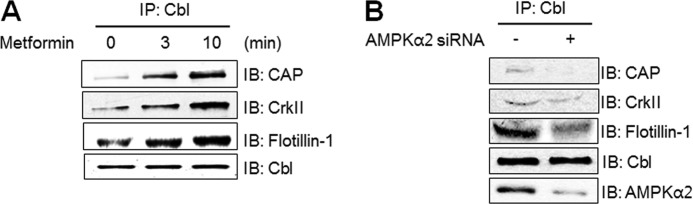
Formation of the Cbl/CAP Complex Was Regulated by AMPK Activation
To corroborate the role of the Cbl/CAP complex in the AMPK-mediated signaling pathway, we assessed the interaction between Cbl and CAP. Immunoprecipitation with antibody for Cbl revealed that Cbl associated easily with proteins such as CAP, CrkII, and flotillin-1 under metformin stimulation (Fig. 5A). To verify the role of AMPK on protein complexes, we assessed the interaction among Cbl and other proteins under the AMPKα2 knockdown condition. The interaction between Cbl and CAP decreased under the AMPKα2 knockdown conditions (Fig. 5B). These results indicate that AMPKα2 has a regulatory function in the Cbl/CAP complex formation.
FIGURE 5.
Formation of the Cbl/CAP complex was regulated by AMPK activation. A, 3T3-L1 preadipocytes were treated with 10 mm metformin for the indicated times and then were immunoprecipitated (IP) with anti-Cbl antibody, followed by Western blotting (IB) with anti-CAP or anti-CrkII, anti-flotillin-1, and anti-Cbl antibodies. B, 3T3-Ll preadipocytes were transiently transfected with AMPKα2 siRNA for 48 h. Cell lysates were immunoprecipitated with the anti-Cbl antibody, followed by Western blotting with anti-CAP or anti-CrkII, anti-flotillin-1, anti-AMPKα2, and anti-Cbl antibodies.
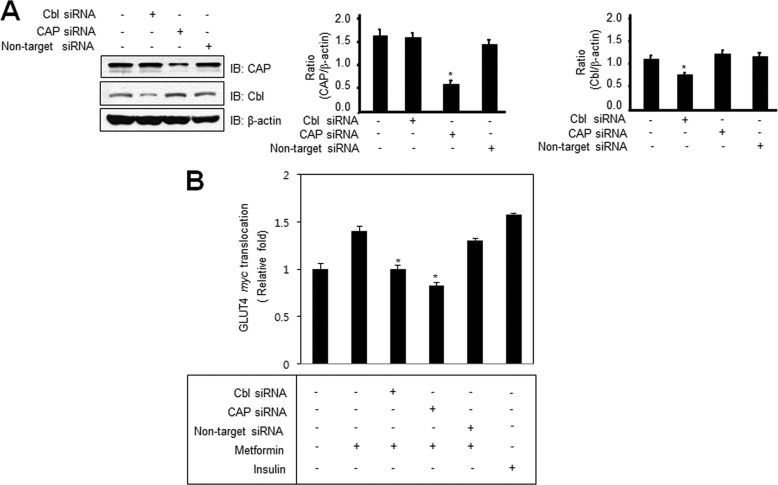
Knockdown of Cbl and CAP Blocks Metformin-induced GLUT4 Translocation
To verify the roles of Cbl and CAP in metformin-mediated GLUT4 signaling, we examined the effects of Cbl and CAP knockdown on GLUT4 translocation. First, we showed that Cbl and CAP siRNA knockdown decreased the Cbl and CAP expression, whereas nontarget siRNA did not produce such an effect (Fig. 6A). Because immunofluorescent microscopy did not provide accurate quantitative analysis of GLUT4 translocation, we used colorimetric assay to measure accurately the level of cell surface GLUT4myc. It is interesting to note that there was a decrease in translocation of GLUT4 to the plasma membranes either Cbl or CAP knockdown cells (Fig. 6B). Insulin was used as a positive control for GLUT4 translocation. These results indicate that Cbl and CAP are key molecules for the metformin-mediated GLUT4 translocation.
FIGURE 6.
Knockdown of Cbl and CAP blocks metformin-induced GLUT4 translocation. A, 3T3-L1 preadipocytes were transiently transfected with CAP, Cbl, or nontarget siRNA for 48 h. Cell lysates (25 μg) were analyzed by Western blotting (IB) with the anti-CAP or anti-Cbl antibody. Blotting with the anti-β-actin antibody was used as a protein-loading control. B, L6-GLUT4myc-tagged cells were transfected with CAP or Cbl siRNA for 48 h. Cells were then treated with 10 mm metformin for 30 min. Cell surface GLUT4myc levels on intact cells were determined as described under “Experimental Procedures.” Each value is expressed as the mean ± S.E. (error bars) of three independent determinants. *, p < 0.05 versus metformin-treated condition.
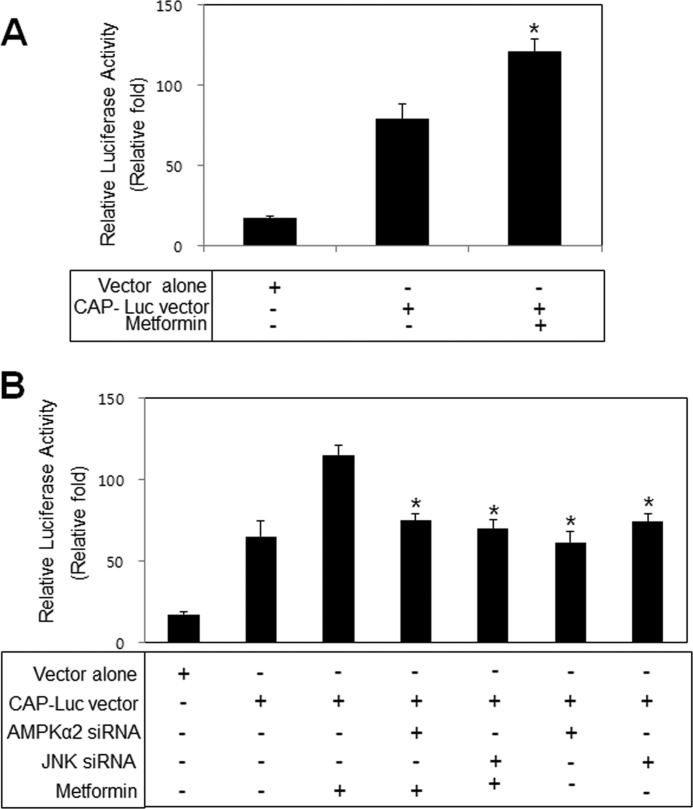
Metformin Stimulates CAP Promoter in a JNK- and AMPK-dependent Manner
To characterize the molecular mechanisms of CAP induction, we evaluated the effects of metformin on the promoter activity of CAP. Metformin stimulated the promoter activity of CAP (Fig. 7A). To evaluate the question of whether AMPK and JNK regulate CAP transcription, we examined the effects of metformin on the promoter activity of CAP when AMPKα2 and JNK were down-regulated. Knockdown of AMPKα2 and JNK decreased metformin-induced CAP promoter activation (Fig. 7B), suggesting that AMPK and JNK are involved in metformin-induced CAP induction. These results demonstrate that metformin plays a stimulatory role with respect to the CAP promoter in an AMPK- and JNK-dependent manner.
FIGURE 7.
Metformin stimulates CAP promoter in a JNK- and AMPK-dependent manner. A, promoter analysis of CAP in 3T3-L1 preadipocytes is shown. Cells were stimulated for 30 min with 10 mm metformin. Promoter activity was then measured. *, p < 0.05 versus CAP-Luc vector. B, cells were transiently transfected with AMPKα2 and JNK siRNA. After 48 h, cells were treated with 10 mm metformin for 30 min. Promoter activity was then measured. Each value is expressed as the mean ± S.E. (error bars) of three independent determinants. *, p < 0.05 versus metformin-treated in CAP vector transfection.
DISCUSSION
The principal finding of this study was that AMPK is involved in GLUT4 translocation. Specifically, we demonstrated that Cbl/CAP is instrumental in AMPK-mediated GLUT4 translocation.
The primary outcome of this study is that in 3T3-L1 preadipocytes AMPK mediates the translocation of GLUT4 through a Cbl/CAP pathway. The anti-diabetes mellitus role of AMPK has previously been evaluated in conjunction with an insulin signal modulator (30, 31) and GLUT4 (32, 33). The GLUT4-modulating properties of AMPK appear to be responsible for its role as an insulin signal modulator and may also contribute to its observed anti-diabetes mellitus effects. Stimulation of glucose transport by insulin involves the tyrosine phosphorylation of Cbl and translocation of the Cbl/CAP complex to lipid raft subdomains of the plasma membrane (26). Enhancement of insulin sensitivity and amelioration of insulin resistance by AMPK is well established (34, 35). However, the interaction between AMPK and insulin has not been fully elucidated. The net effect of AMPK on insulin signaling is complex and involves multiple targets, depending on the cellular context. In the present study, we demonstrated that short term treatment with metformin increased phosphorylation of Cbl, and chronic treatment with metformin induced expression of CAP through an AMPK-dependent pathway. We also explored the mechanisms by which AMPK enhances insulin sensitivity. Collectively, our results indicate that Cbl/CAP may play crucial roles in the cross-talk between AMPK and insulin signaling.
The second objective of the present study was to ascertain whether or not Cbl/CAP is directly regulated by metformin and, if so, to determine which molecules are involved in this process. Our data revealed a novel role of JNK downstream of AMPK activation by metformin. Our identification of an AMPK-JNK-CAP axis in metformin-treated 3T3-L1 preadipocytes led us to hypothesize that CAP plays a role in AMPK-mediated modulation of insulin signaling. Indeed, this is the first report demonstrating a link between AMPK and CAP in 3T3-L1 preadipocytes. We further demonstrated that metformin plays a metabolic role through activation of CAP. Furthermore, our data showed that phosphorylation of Cbl at Tyr731 was increased through an AMPK-dependent pathway. This result may be explained by the interaction with Src. Therefore, AMPK is able to enhance the interaction between Cbl and CAP by regulation of the JNK pathway. Overall, although the mechanism by which AMPK influences insulin signaling remains unknown, the findings of this report suggest that metformin may induce Cbl/CAP through the AMPK pathway as part of the process of metformin-mediated insulin signal modulation.
Ribon et al. (26) and Kimura et al. (36) have reported that insulin stimulates the interaction between CAP and Cbl by Cbl phosphorylation. The Cbl/CAP complex migrates to caveolin-enriched lipid rafts, due to the interaction between the SoHo domain on CAP and the lipid raft-associated protein flotillin-1 (26, 37). This leads to recruitment of the CrkII/C3G complex to the microdomain of the plasma membrane, where C3G, a guanyl nucleotide exchange factor, activates the small G protein TC10 (29). Insulin triggers translocation of GLUT4 to the plasma membrane by regulating the interaction between CAP and Cbl. In this study, we found that AMPK stimulates not only the interaction between Cbl and CAP but also the level of the CAP protein. This result indicates that the AMPK pathway may trigger translocation of GLUT4 by regulating the amount of the Cbl/CAP multicomplex.
This work was supported by the National Research Foundation of Korea funded by Korean Government Grant 2010-0011053.
- GLUT4
- glucose transport 4
- AICAR
- 5-aminoimidazole-4-carboxy-amide-1-d-ribofuranoside
- AMPK
- AMP-activated protein kinase
- CAP
- Cbl-associated protein
- SH
- Src homology.
REFERENCES
- 1. Witters L. A. (2001) The blooming of the French lilac. J. Clin. Invest. 108, 1105–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meuillet E. J., Wiernsperger N., Mania-Farnell B., Hubert P., Cremel G. (1999) Metformin modulates insulin receptor signaling in normal and cholesterol-treated human hepatoma cells (HepG2). Eur. J. Pharmacol. 377, 241–252 [DOI] [PubMed] [Google Scholar]
- 3. Kumar N., Dey C. S. (2002) Metformin enhances insulin signalling in insulin-dependent and -independent pathways in insulin-resistant muscle cells. Br. J. Pharmacol. 137, 329–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gunton J. E., Delhanty P. J., Takahashi S., Baxter R. C. (2003) Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J. Clin. Endocrinol. Metab. 88, 1323–1332 [DOI] [PubMed] [Google Scholar]
- 5. Yang J., Holman G. D. (2006) Long-term metformin treatment stimulates cardiomyocyte glucose transport through an AMP-activated protein kinase-dependent reduction in GLUT4 endocytosis. Endocrinology 147, 2728–2736 [DOI] [PubMed] [Google Scholar]
- 6. Sarabia V., Lam L., Burdett E., Leiter L. A., Klip A. (1992) Glucose transport in human skeletal muscle cells in culture: stimulation by insulin and metformin. J. Clin. Invest. 90, 1386–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fischer Y., Thomas J., Rösen P., Kammermeier H. (1995) Action of metformin on glucose transport and glucose transporter GLUT1 and GLUT4 in heart muscle cells from healthy and diabetic rats. Endocrinology 136, 412–420 [DOI] [PubMed] [Google Scholar]
- 8. Hardie D. G., Carling D. (1997) The AMP-activated protein kinase: fuel gauge of the mammalian cell? Eur. J. Biochem. 246, 259–273 [DOI] [PubMed] [Google Scholar]
- 9. Makinde A. O., Gamble J., Lopaschuk G. D. (1997) Up-regulation of 5′-AMP-activated protein kinase is responsible for the increase in myocardial fatty acid oxidation rates following birth in the newborn rabbit. Circ. Res. 80, 482–489 [DOI] [PubMed] [Google Scholar]
- 10. Ai H., Ihlemann J., Hellsten Y., Lauritzen H. P., Hardie D. G., Galbo H., Ploug T. (2002) Effect of fiber type and nutritional state on AICAR- and contraction-stimulated glucose transport in rat muscle. Am. J. Physiol. Endocrinol. Metab. 282, E1291–1300 [DOI] [PubMed] [Google Scholar]
- 11. Zong H., Ren J. M., Young L. H., Pypaert M., Mu J., Birnbaum M. J., Shulman G. I. (2002) AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. U.S.A. 99, 15983–15987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henin N., Vincent M. F., Gruber H. E., Van den Berghe G. (1995) Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J. 9, 541–546 [DOI] [PubMed] [Google Scholar]
- 13. Hayashi T., Hirshman M. F., Kurth E. J., Winder W. W., Goodyear L. J. (1998) Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47, 1369–1373 [DOI] [PubMed] [Google Scholar]
- 14. Mu J., Brozinick J. T., Jr., Valladares O., Bucan M., Birnbaum M. J. (2001) A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell 7, 1085–1094 [DOI] [PubMed] [Google Scholar]
- 15. Huang S., Czech M. P. (2007) The GLUT4 glucose transporter. Cell Metab. 5, 237–252 [DOI] [PubMed] [Google Scholar]
- 16. Shepherd P. R., Withers D. J., Siddle K. (1998) Phosphoinositide 3-kinase: the key switch mechanism in insulin signaling. Biochem. J. 333, 471–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tanti J. F., Grillo S., Grémeaux T., Coffer P. J., Van Obberghen E., Le Marchand-Brustel Y. (1997) Potential role of protein kinase B in glucose transporter 4 translocation in adipocytes. Endocrinology 138, 2005–2010 [DOI] [PubMed] [Google Scholar]
- 18. Ueki K., Yamamoto-Honda R., Kaburagi Y., Yamauchi T., Tobe K., Burgering B. M., Coffer P. J., Komuro I., Akanuma Y., Yazaki Y., Kadowaki T. (1998) Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J. Biol. Chem. 273, 5315–5322 [DOI] [PubMed] [Google Scholar]
- 19. Alessi D. R., James S. R., Downes C. P., Holmes A. B., Gaffney P. R., Reese C. B., Cohen P. (1997) Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7, 261–269 [DOI] [PubMed] [Google Scholar]
- 20. Blake T. J., Shapiro M., Morse H. C., 3rd, Langdon W. Y. (1991) The sequences of the human and mouse c-cbl proto-oncogenes show v-cbl was generated by a large truncation encompassing a proline-rich domain and a leucine zipper-like motif. Oncogene 6, 653–657 [PubMed] [Google Scholar]
- 21. Liu J., Kimura A., Baumann C. A., Saltiel A. R. (2002) APS facilitates c-Cbl tyrosine phosphorylation and GLUT4 translocation in response to insulin in 3T3-L1 adipocytes. Mol. Cell. Biol. 22, 3599–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thien C. B., Langdon W. Y. (2001) Cbl: many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell Biol. 2, 294–307 [DOI] [PubMed] [Google Scholar]
- 23. Liu J., DeYoung S. M., Hwang J. B., O'Leary E. E., Saltiel A. R. (2003) The roles of Cbl-b and c-Cbl in insulin-stimulated glucose transport. J. Biol. Chem. 278, 36754–36762 [DOI] [PubMed] [Google Scholar]
- 24. Ribon V., Printen J. A., Hoffman N. G., Kay B. K., Saltiel A. R. (1998) A novel, multifunctional c-Cbl-binding protein in insulin receptor signaling in 3T3-L1 adipocytes. Mol. Cell. Biol. 18, 872–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alcazar O., Ho R. C., Fujii N., Goodyear L. J. (2004) cDNA cloning and functional characterization of a novel splice variant of c-Cbl-associated protein from mouse skeletal muscle. Biochem. Biophys. Res. Commun. 317, 285–293 [DOI] [PubMed] [Google Scholar]
- 26. Ribon V., Herrera R., Kay B. K., Saltiel A. R. (1998) A role for CAP, a novel, multifunctional Src homology 3 domain-containing protein in formation of actin stress fibers and focal adhesions. J. Biol. Chem. 273, 4073–4080 [DOI] [PubMed] [Google Scholar]
- 27. Baumann C. A., Chokshi N., Saltiel A. R., Ribon V. (2000) Cloning and characterization of a functional peroxisome proliferator activator receptor-γ-responsive element in the promoter of the CAP gene. J. Biol. Chem. 275, 9131–9135 [DOI] [PubMed] [Google Scholar]
- 28. Wijesekara N., Tung A., Thong F., Klip A. (2006) Muscle cell depolarization induces a gain in surface GLUT4 via reduced endocytosis independently of AMPK. Am. J. Physiol. Endocrinol. Metab. 290, E1276–1286 [DOI] [PubMed] [Google Scholar]
- 29. Chiang S. H., Baumann C. A., Kanzaki M., Thurmond D. C., Watson R. T., Neudauer C. L., Macara I. G., Pessin J. E., Saltiel A. R. (2001) Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 410, 944–948 [DOI] [PubMed] [Google Scholar]
- 30. Longnus S. L., Ségalen C., Giudicelli J., Sajan M. P., Farese R. V., Van Obberghen E. (2005) Insulin signalling downstream of protein kinase B is potentiated by 5′AMP-activated protein kinase in rat hearts in vivo. Diabetologia 48, 2591–2601 [DOI] [PubMed] [Google Scholar]
- 31. Jessen N., Pold R., Buhl E. S., Jensen L. S., Schmitz O., Lund S. (2003) Effects of AICAR and exercise on insulin-stimulated glucose uptake, signaling, and GLUT-4 content in rat muscles. J. Appl. Physiol. 94, 1373–1379 [DOI] [PubMed] [Google Scholar]
- 32. Yamaguchi S., Katahira H., Ozawa S., Nakamichi Y., Tanaka T., Shimoyama T., Takahashi K., Yoshimoto K., Imaizumi M. O., Nagamatsu S., Ishida H. (2005) Activators of AMP-activated protein kinase enhance GLUT4 translocation and its glucose transport activity in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 289, E643–649 [DOI] [PubMed] [Google Scholar]
- 33. Kurth-Kraczek E. J., Hirshman M. F., Goodyear L. J., Winder W. W. (1999) 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 48, 1667–1671 [DOI] [PubMed] [Google Scholar]
- 34. Winder W. W., Hardie D. G. (1999) AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am. J. Physiol. 277, E1–10 [DOI] [PubMed] [Google Scholar]
- 35. Barnes B. R., Zierath J. R. (2005) Role of AMP-activated protein kinase in the control of glucose homeostasis. Curr. Mol. Med. 5, 341–348 [DOI] [PubMed] [Google Scholar]
- 36. Kimura A., Baumann C. A., Chiang S. H., Saltiel A. R. (2001) The sorbin homology domain: a motif for the targeting of proteins to lipid rafts. Proc. Natl. Acad. Sci. U.S.A. 98, 9098–9103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baumann C. A., Ribon V., Kanzaki M., Thurmond D. C., Mora S., Shigematsu S., Bickel P. E., Pessin J. E., Saltiel A. R. (2000) CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 407, 202–207 [DOI] [PubMed] [Google Scholar]