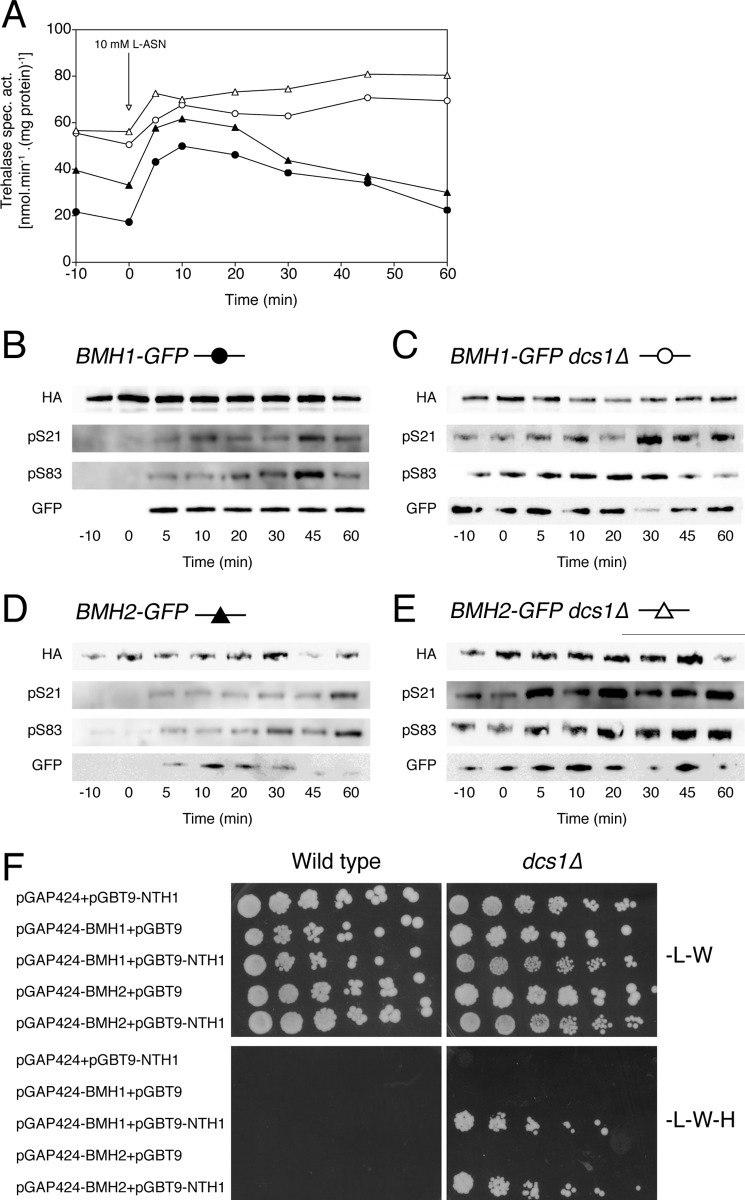
FIGURE 7.
Nitrogen-induced trehalase activation and phosphorylation in the dcs1Δ strain. Trehalase activity (A) and phosphorylation status of Ser21 (pS21) and Ser83 (pS83) in trehalase (B–E) after the addition of 10 mm l-asparagine to fermenting, nitrogen-starved yeast cells. Wild type and dcs1Δ cells, expressing Bmh1-GFP or Bmh2-GFP, were transformed with pTPI1-NTH1-HA-LEU2. Trehalase was immunoprecipitated, and phosphorylation of Ser21 and Ser83 was visualized with phosphospecific Abs (pS21 and pS83). In addition, anti-GFP Ab was used to visualize any interacting Bmh proteins (GFP). F, two-hybrid analysis of direct physical interaction between Nth1 and the 14-3-3 proteins, Bmh1 and Bmh2. Two-hybrid reporter strains PJ69-4A wild type and PJ69-4A dcs1Δ, transformed with the indicated plasmids, expressing Nth1, Bmh1, and/or Bmh2, were grown overnight in liquid selective medium (SD−Leu−Trp) and spotted on selective SD−Leu−Trp (−L−W) and SD−Leu−Trp−His (−L−W−H) media.