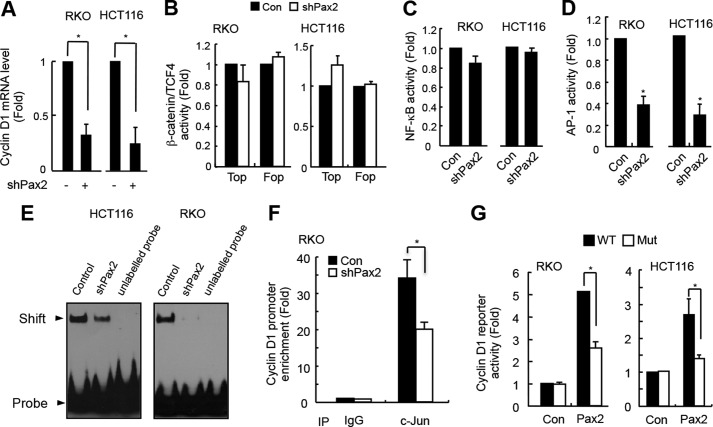
FIGURE 3.
PAX2 induces cyclin D1 through AP-1. A, knockdown of PAX2 decreased cyclin D1 mRNA level. HCT116 and RKO cells were infected with control or shPAX2 adenovirus. In 48 h, the cells were harvested for real-time PCR analysis. B, PAX2 did not affect the transcriptional activity of β-catenin/TCF4. HCT116 and RKO cells were transfected with TOP/FOP reporter and β-galactosidase plasmids. The next day, the cells were infected with control (Con) or shPAX2 adenovirus. In 48 h, the luciferase activity was determined. C, PAX2 did not affect NF-κB activity. HCT116 and RKO cells were transfected with NF-κB reporter and β-galactosidase plasmids. The cells were incubated overnight followed by infection with control or shPAX2 adenovirus. In 48 h, the relative NF-κB activity was determined by measuring luciferase activity. D, knockdown of PAX2 repressed AP-1 activity. HCT116 and RKO cells were transfected with AP-1 reporter and β-galactosidase plasmids. The transfected cells were incubated overnight and then infected with control or shPAX2 adenovirus. In 48 h, the cells were harvested for luciferase assay. E, knockdown of PAX2 inhibited AP-1 DNA binding activity. HCT116 and RKO cells were infected with control or shPAX2 adenovirus. In 72 h, the infected cells were harvested for EMSA assay. F, ChIP assay was performed as described under “Materials and Methods.” RKO cells were used in the experiment. G, PAX2 regulated expression of cyclin D1 via AP-1. The cyclin D1 promoter reporter and mutated cyclin D1 promoter reporter plasmids were prepared as described under “Materials and Methods.” RKO and HCT116 cells were transfected with cyclin D1 promoter reporter, PAX2, and β-galactosidase plasmids. In 24 h, the cells were harvested for luciferase activity assay. *, p < 0.05 versus control (n = 3).