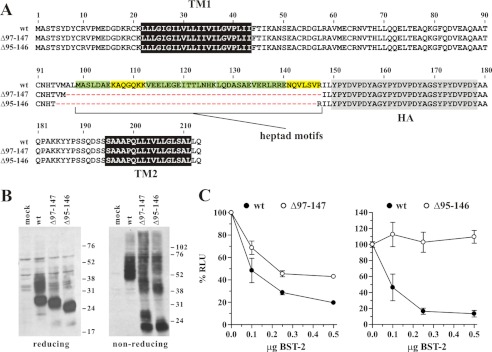
FIGURE 5.
Heptad motifs in the C-terminal coiled-coil region of the BST-2 ectodomain are dispensable for tetherin function. A, amino acid alignment of BST-2 deletions Δ97–147 and Δ95–146 as compared with BST-2 wt. Heptad motifs are highlighted. Green highlights indicate motifs that perfectly match the HXXHEXE consensus motif where H is hydrophobic or uncharged and E is a charged residue; yellow highlights depict motifs in which one residue in the heptad sequence does not match the canonical motif. Black and shaded areas represent the transmembrane domains and the HA tag, respectively. B, expression and dimerization of BST-2 variants was tested as described in the legend to Fig. 1B. C, 293T cells were transfected with 5 μg each of NL4–3/Udel together with varying amounts of BST-2 wt (closed circles) or mutant DNA (open circles) as indicated. Virus-containing supernatants were collected 24 h later and used to infect HeLa TZM-bl indicator cells. Analysis of the data were performed as described in the legend to Fig. 1C. Values are expressed as mean ± S.E. of nine experiments. The signal produced by NL4–3/Udel virus in the absence of BST-2 was defined as 100%.